Carbon and the Molecular Diversity of Life Organic
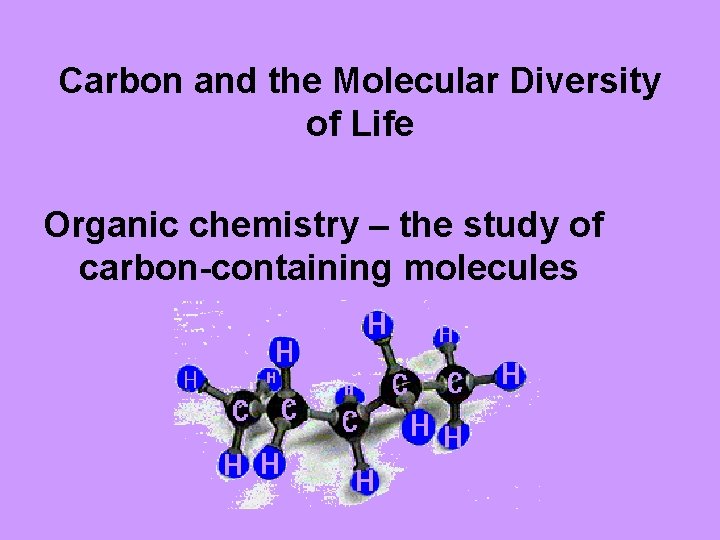
Carbon and the Molecular Diversity of Life Organic chemistry – the study of carbon-containing molecules

With a total of 6 electrons, a carbon atom has 2 in the first shell and 4 in the second shell • carbon has little tendency to form ionic bonds by loosing or gaining 4 electrons
• carbon usually completes its valence shell by sharing electrons with other atoms in four covalent bonds • this tetravalence by carbon makes large, complex molecules possible
• it is this tetravalence that allow carbon to form large and complex molecules with characteristic 3 -D shapes and properties • carbon atoms readily bond with each other, producing chains or rings of carbon atoms
The simplest organic molecules are hydrocarbons • consisting of only carbon and hydrogen • the nonpolar C-H bonds account for their hydrophobic behavior
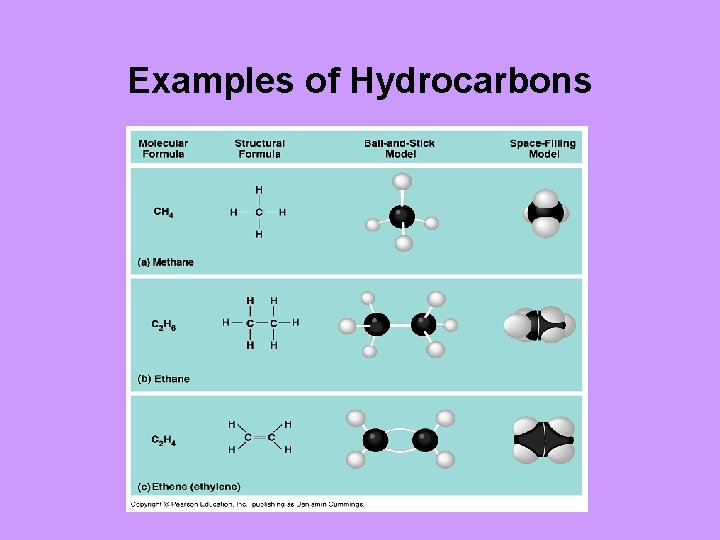
Examples of Hydrocarbons
Isomers are compounds with the same molecular formula but different structural arrangements • they have different molecular and chemical properties
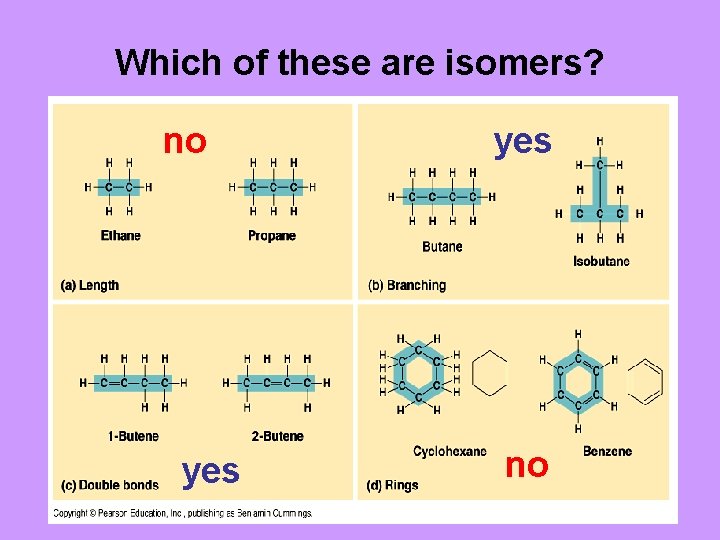
Which of these are isomers? no yes no
Types Of Isomers 1. Structural 2. Geometric 3. Enantiomers
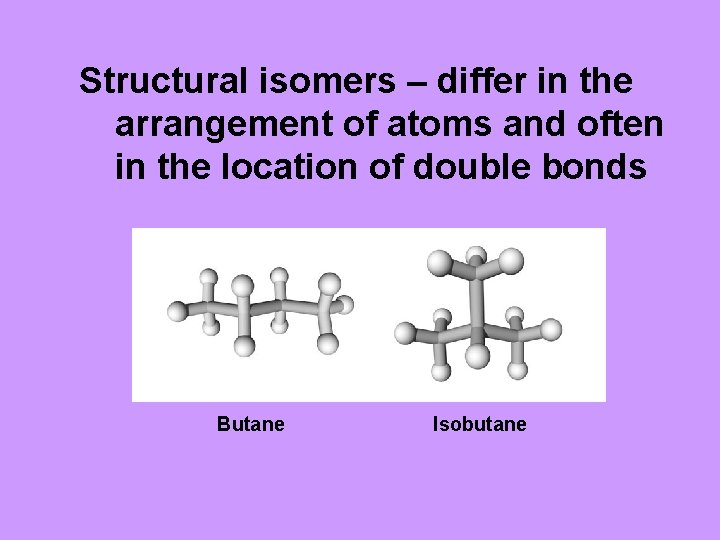
Structural isomers – differ in the arrangement of atoms and often in the location of double bonds Butane Isobutane
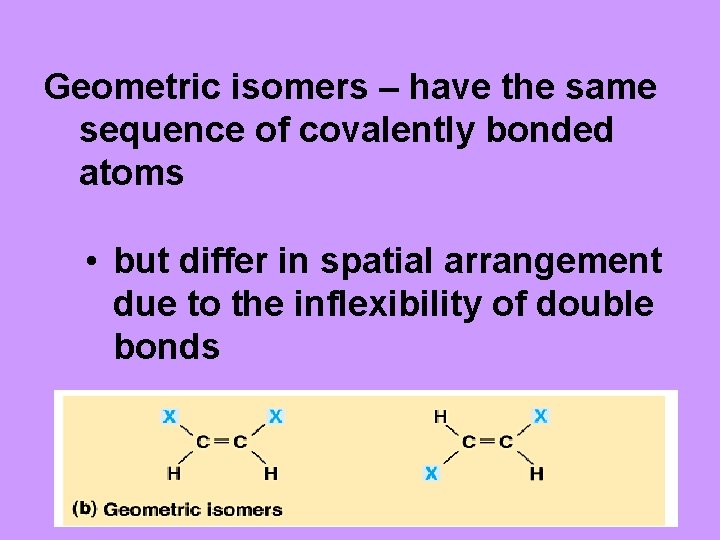
Geometric isomers – have the same sequence of covalently bonded atoms • but differ in spatial arrangement due to the inflexibility of double bonds
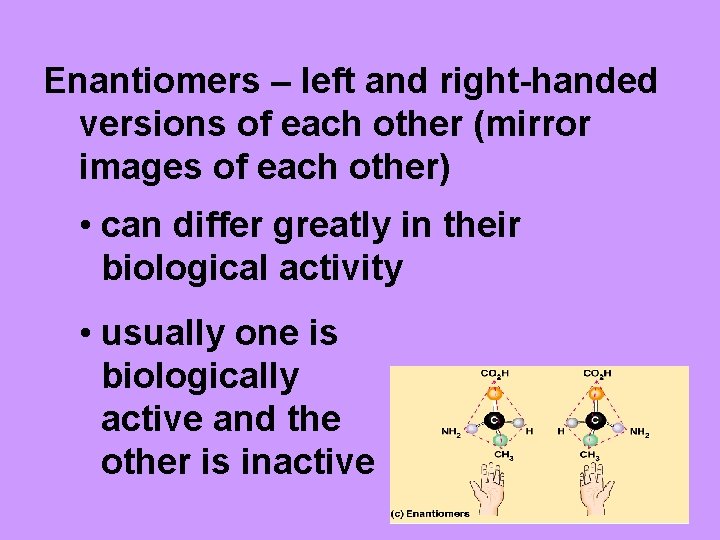
Enantiomers – left and right-handed versions of each other (mirror images of each other) • can differ greatly in their biological activity • usually one is biologically active and the other is inactive
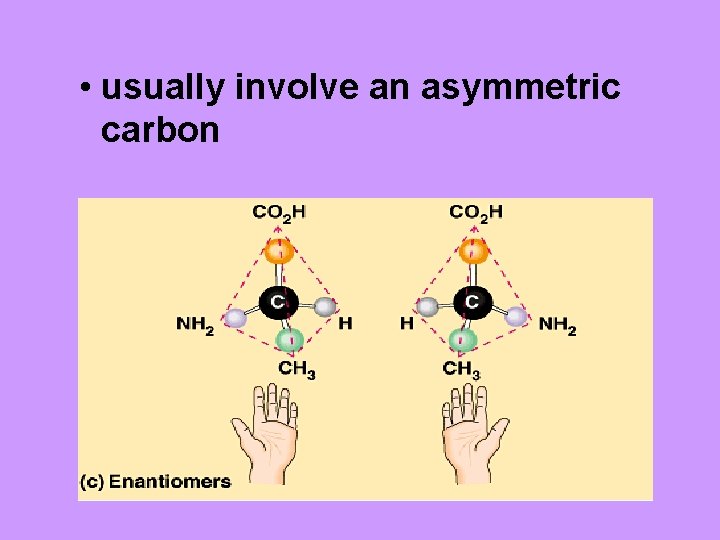
• usually involve an asymmetric carbon

Organisms are sensitive to even the most subtle variations in molecular architecture Example - Thalidomide • one enantiomer of this drug reduced morning sickness but the other isomer caused severe birth defects

Carbon, oxygen, hydrogen, nitrogen, and smaller quantities of sulfur and phosphorus, all capable of forming strong covalent bonds, are combined into the complex organic molecules of living matter

The versatility of carbon in forming 4 covalent bonds, linking readily with itself to produce chains and rings, and binding with other elements and functional groups makes possible the diversity of organic molecules (carbohydrates, lipids, proteins, and nucleic acids)
- Slides: 16