CARBOHYDRATES Very common macromolecule Used for celltocell identification
CARBOHYDRATES ● ● Very common macromolecule Used for cell-to-cell identification and communication, building blocks for larger macromolecules and ENERGY. Empirical formula (CH 2 O)n where the n stands for the number of carbon molecules Three types: monosaccharides, oligosaccharides and polysaccharides.
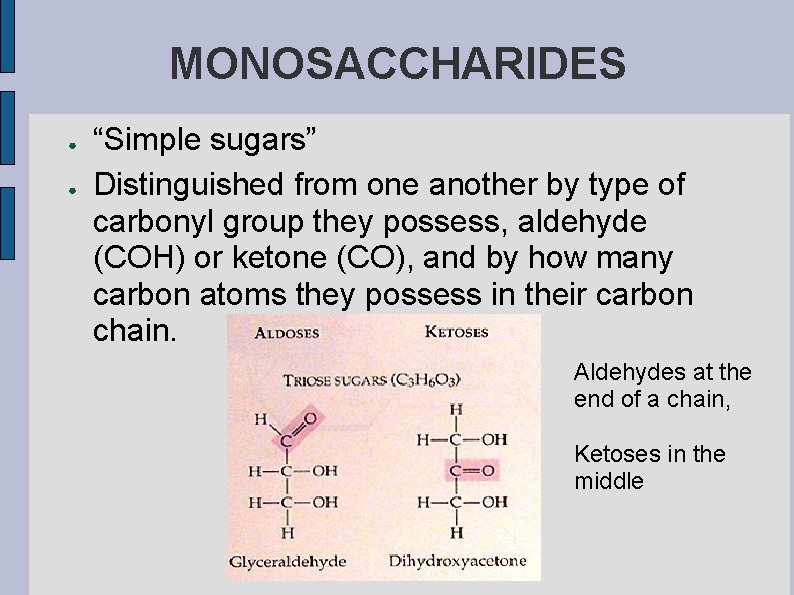
MONOSACCHARIDES ● ● “Simple sugars” Distinguished from one another by type of carbonyl group they possess, aldehyde (COH) or ketone (CO), and by how many carbon atoms they possess in their carbon chain. Aldehydes at the end of a chain, Ketoses in the middle
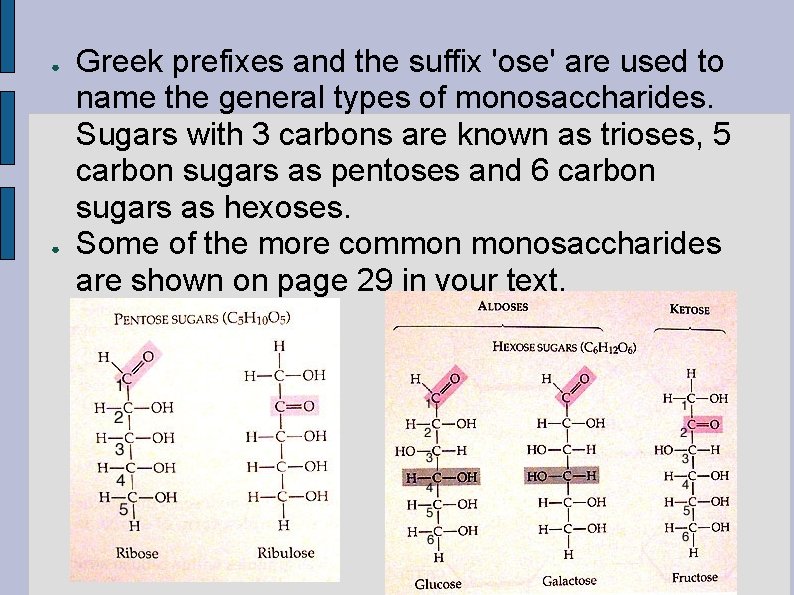
● ● Greek prefixes and the suffix 'ose' are used to name the general types of monosaccharides. Sugars with 3 carbons are known as trioses, 5 carbon sugars as pentoses and 6 carbon sugars as hexoses. Some of the more common monosaccharides are shown on page 29 in your text.

● The hexoses glucose, galactose and fructose are all isomers. Molecules with the same chemical formula, but with different spatial arrangement of atoms. Isomers possess different shapes and have
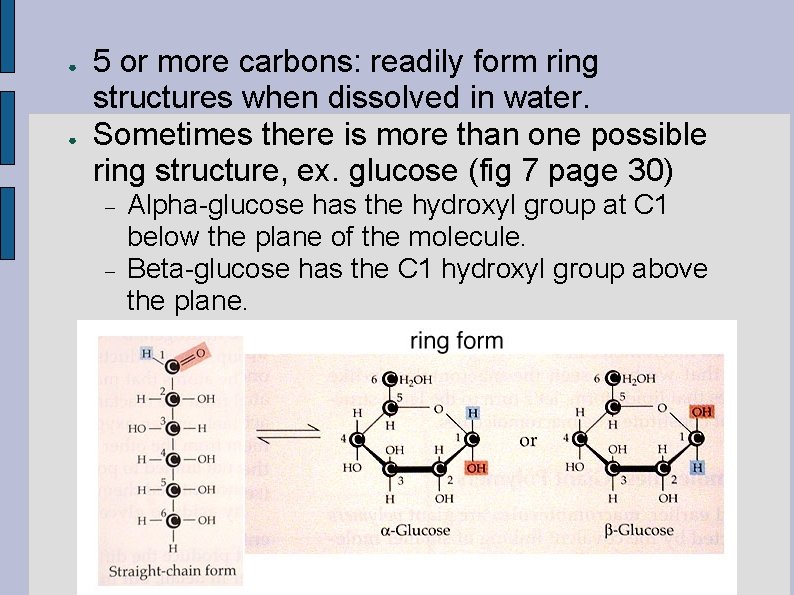
● ● 5 or more carbons: readily form ring structures when dissolved in water. Sometimes there is more than one possible ring structure, ex. glucose (fig 7 page 30) Alpha-glucose has the hydroxyl group at C 1 below the plane of the molecule. Beta-glucose has the C 1 hydroxyl group above the plane.
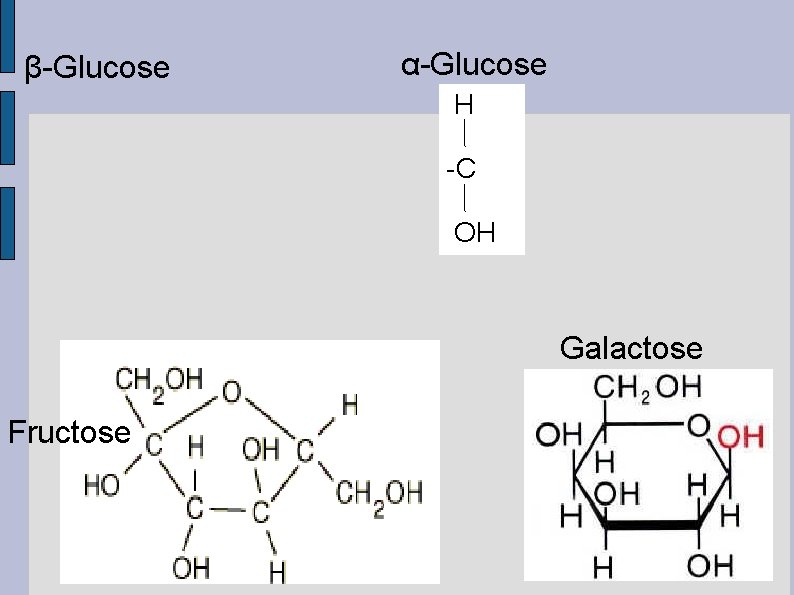
β-Glucose α-Glucose H -C OH Galactose Fructose

OLIGOSACCHARIDES ● ● Contain two or three simple sugars attached together by glycosidic linkages (covalent bonds formed when two monosaccharides undergo a dehydration synthesis reaction). Important oligosaccharides include the disaccharides: Maltose (α-glucose + α-glucose), used in beer Sucrose (α-glucose + α-fructose), table sugar Lactose (α-glucose + α-galactose), milk sugar

● ● The glycosidic linkage in maltose is called an α 1 -4 glycosidic linkage, it occurs between C 1 of the first glucose and C 4 of the second glucose. An α 1 -4 glycosidic linkage occurs in lactose as well between C 1 of the glucose and C 4 of the galactose. The glycosidic linkage in sucrose is called an α 1 -2 glycosidic linkage because it occurs between C 1 of the glucose and C 2 of fructose
POLYSACCHARIDES ● ● Aka “complex carbohydrates” Polymers of hundreds to thousands of monosaccharide molecules held together by glycosidic linkages. Can be straight chains or branched. Used as energy storage (starch, glycogen) and/or structural support (cellulose, chitin)

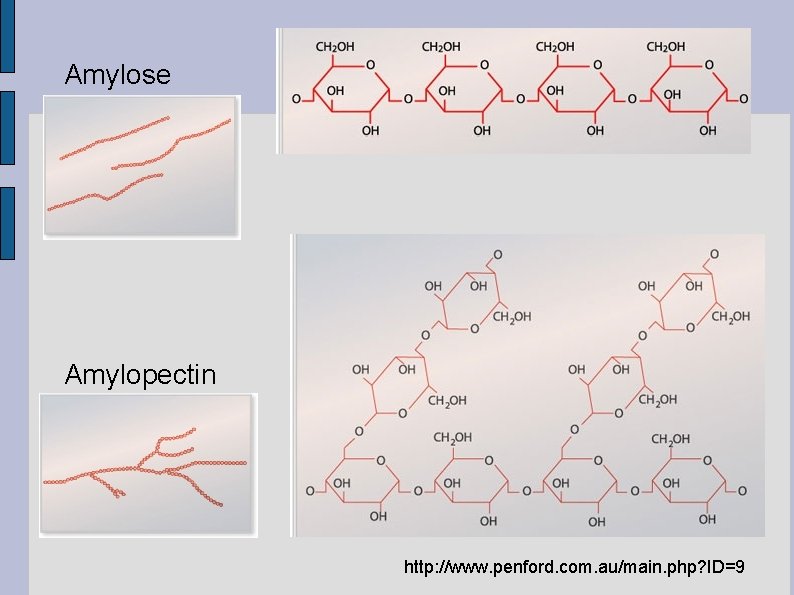
STARCH ● ● Starch is how plants store excess glucose produced during photosynthesis. A mixture of two different polysaccharides: Amylose: a straight chain of α-glucose in α 1 -4 glycosidic linkages, and, Amylopectin: one straight main chain of αglucose in α 1 -4 glycosidic linkages as well as branches of α-glucose connected to the main chain by an α 1 -6 glycosidic linkage (C 1 of the main chain, C 6 of the branch).
Amylose Amylopectin http: //www. penford. com. au/main. php? ID=9

● ● The angles at which the glycosidic linkages form cause the components of starch to coil and become insoluble in water. They are stored in the chloroplast (or other specialized structure) as starch granules. Humans are able to hydrolyze plant starch and break it down into its glucose monomers. Any excess glucose that we don't use immediately for energy is stored as glycogen.
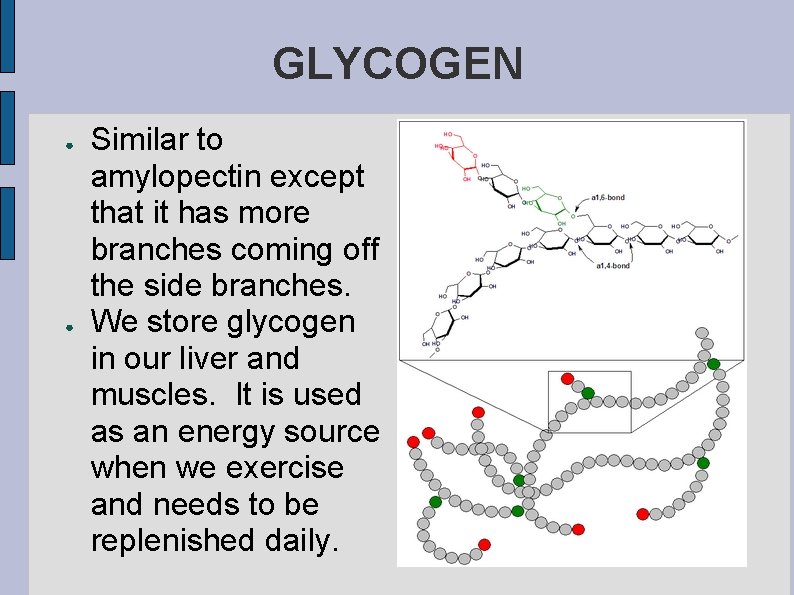
GLYCOGEN ● ● Similar to amylopectin except that it has more branches coming off the side branches. We store glycogen in our liver and muscles. It is used as an energy source when we exercise and needs to be replenished daily.
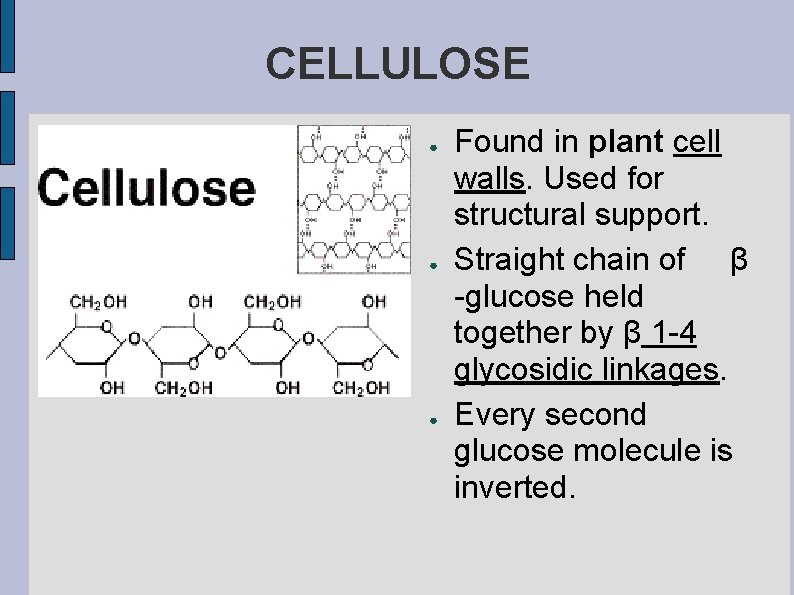
CELLULOSE ● ● ● Found in plant cell walls. Used for structural support. Straight chain of β -glucose held together by β 1 -4 glycosidic linkages. Every second glucose molecule is inverted.

● We cannot digest cellulose, the enzymes in our body are only able to hydrolyze α 1 -4 glycosidic linkages, but cellulose is an important part of our diets because it clears out our digestive tract.
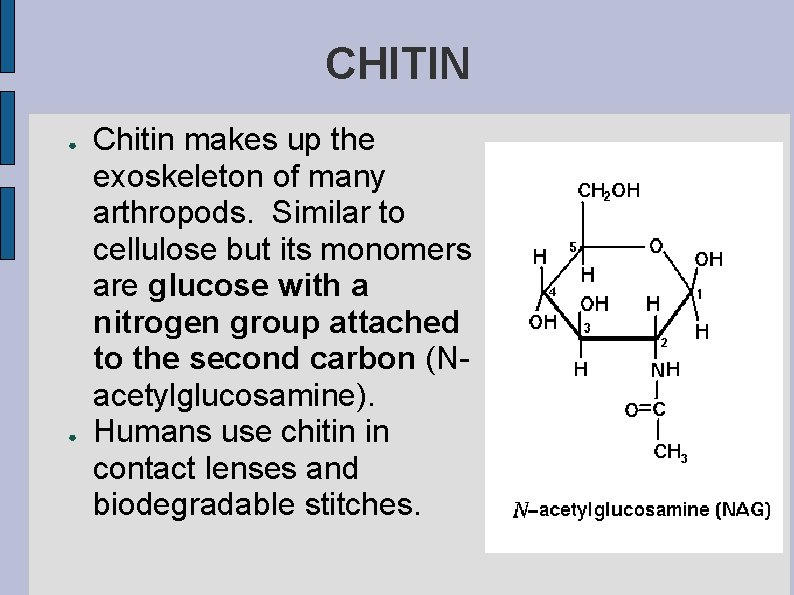
CHITIN ● ● Chitin makes up the exoskeleton of many arthropods. Similar to cellulose but its monomers are glucose with a nitrogen group attached to the second carbon (Nacetylglucosamine). Humans use chitin in contact lenses and biodegradable stitches.
- Slides: 16