CARBOHYDRATES SACCHARIDES SUGARS MONOSACCHARIDES SIMPLE SUGARS PHOTOSYNTHESIS Saccharide

CARBOHYDRATES (SACCHARIDES, SUGARS)

(MONOSACCHARIDES, SIMPLE SUGARS)

PHOTOSYNTHESIS
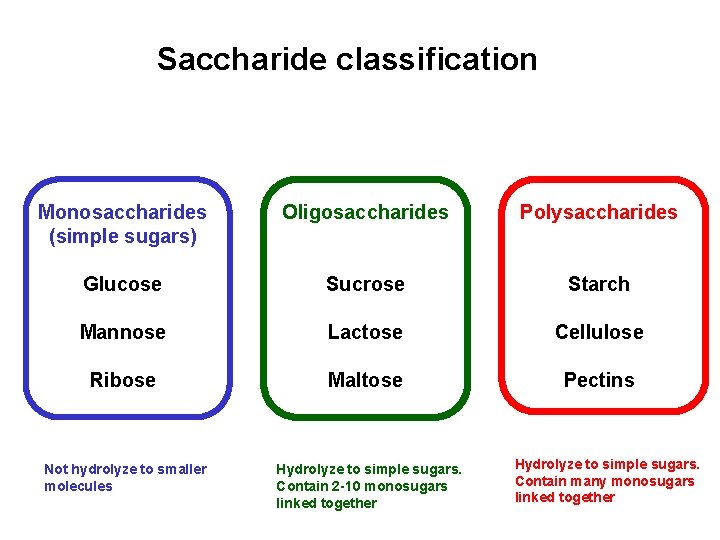
Saccharide classification Monosaccharides (simple sugars) Oligosaccharides Polysaccharides Glucose Sucrose Starch Mannose Lactose Cellulose Ribose Maltose Pectins Not hydrolyze to smaller molecules Hydrolyze to simple sugars. Contain 2 -10 monosugars linked together Hydrolyze to simple sugars. Contain many monosugars linked together
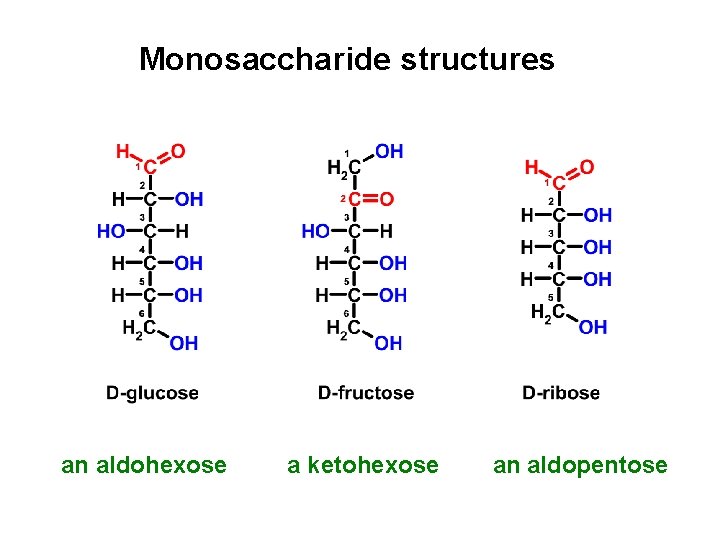
Monosaccharide structures an aldohexose a ketohexose an aldopentose
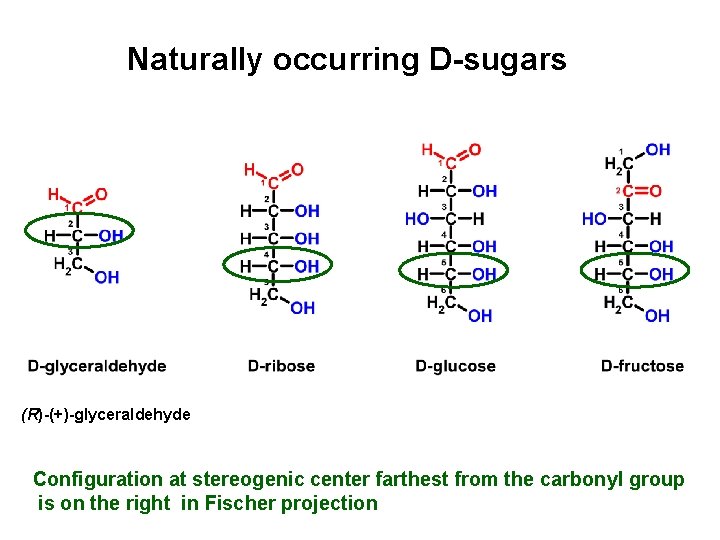
Naturally occurring D-sugars (R)-(+)-glyceraldehyde Configuration at stereogenic center farthest from the carbonyl group is on the right in Fischer projection
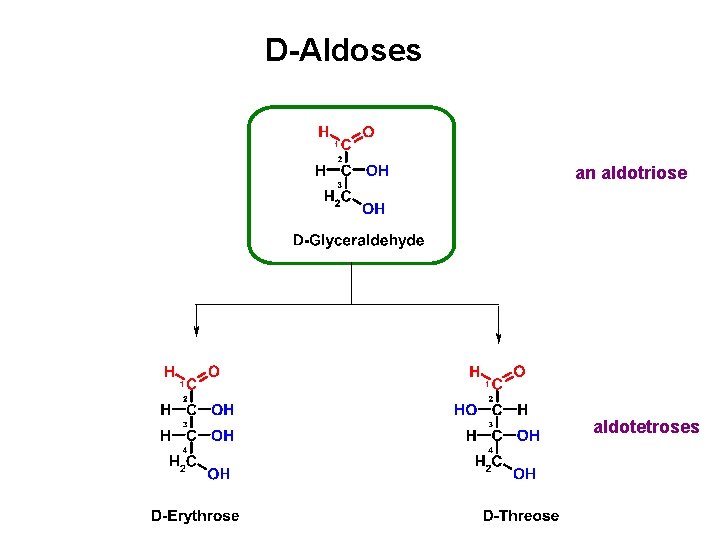
D-Aldoses an aldotriose aldotetroses
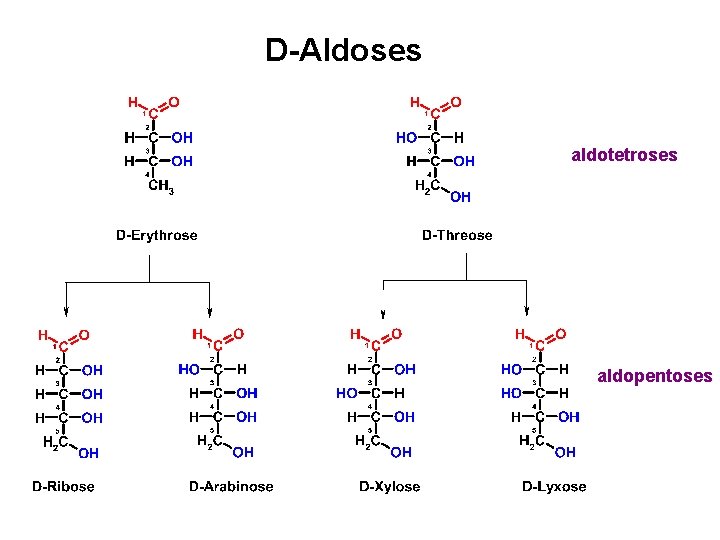
D-Aldoses aldotetroses aldopentoses
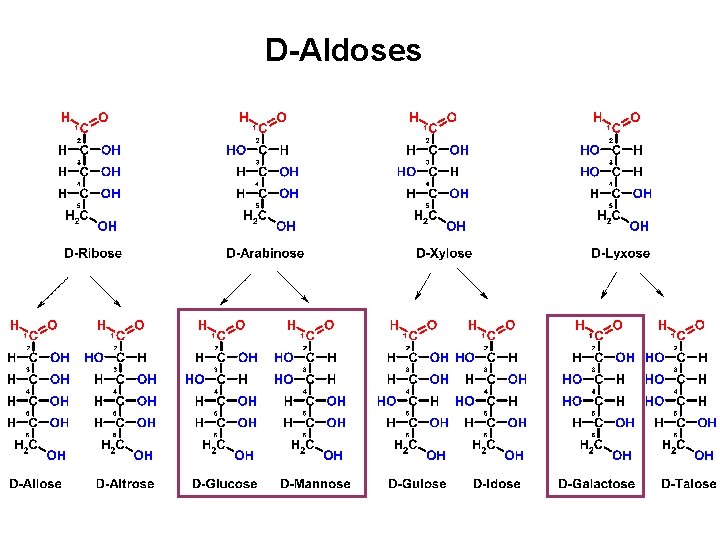
D-Aldoses

D-Aldoses Allose Altrose Glucose Mannose Gulose Idose Galactose Talose All Altruists Gladly Make Gum In Gallon Tanks
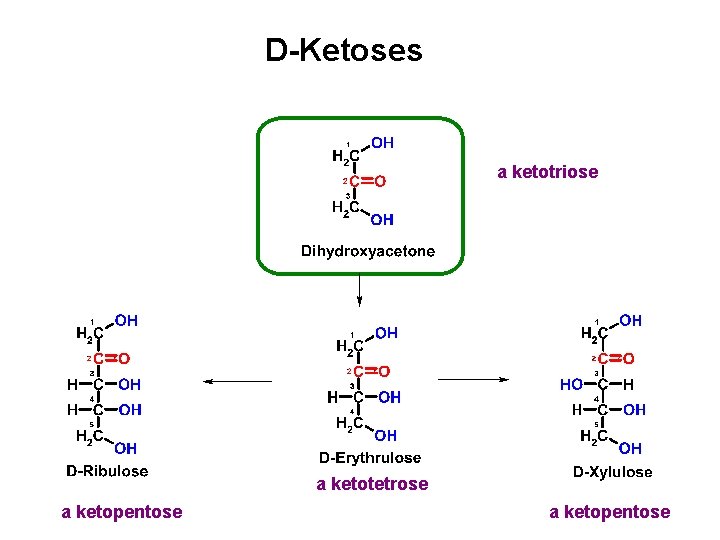
D-Ketoses a ketotriose a ketotetrose a ketopentose
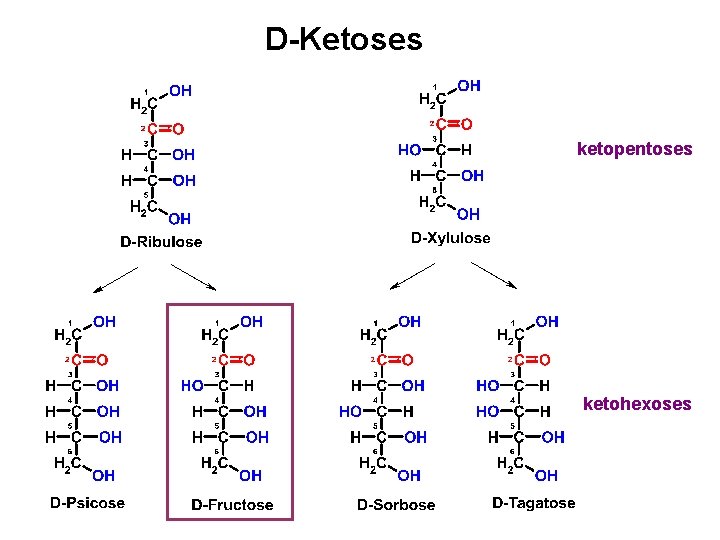
D-Ketoses ketopentoses ketohexoses
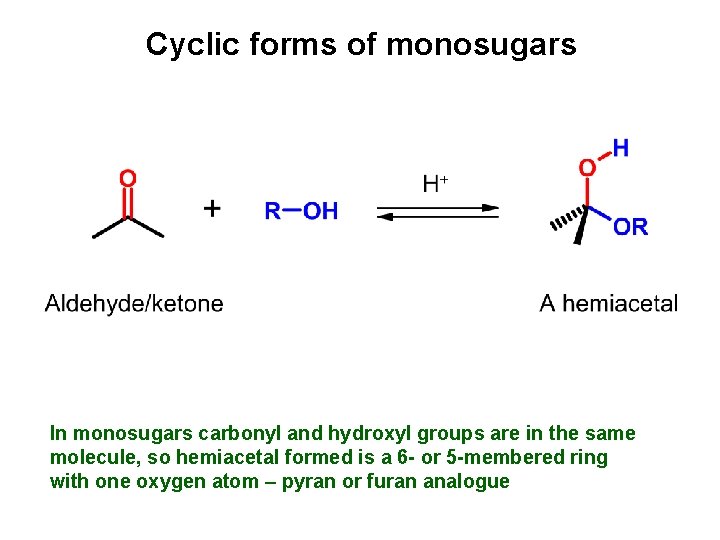
Cyclic forms of monosugars In monosugars carbonyl and hydroxyl groups are in the same molecule, so hemiacetal formed is a 6 - or 5 -membered ring with one oxygen atom – pyran or furan analogue
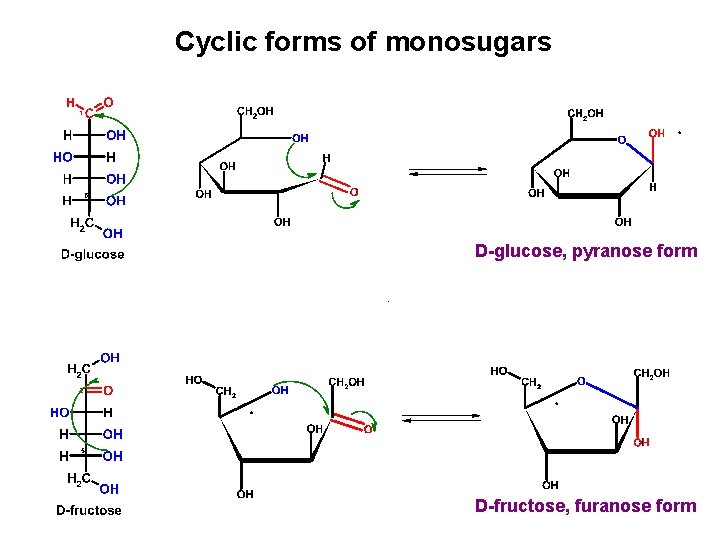
Cyclic forms of monosugars D-glucose, pyranose form D-fructose, furanose form
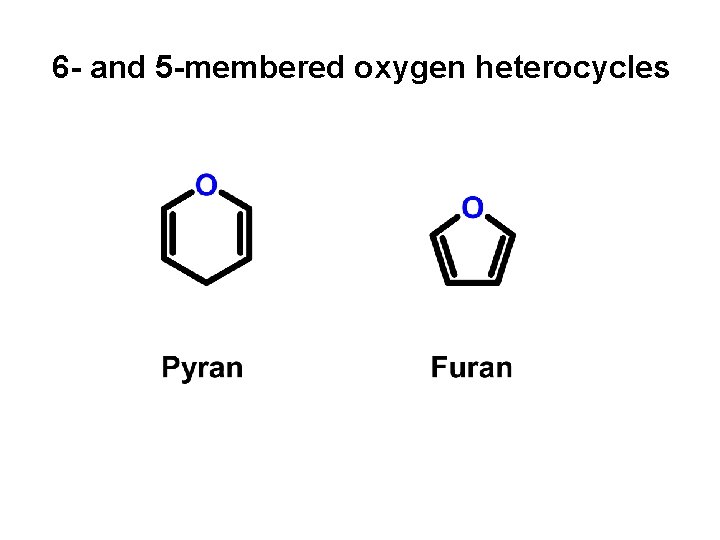
6 - and 5 -membered oxygen heterocycles
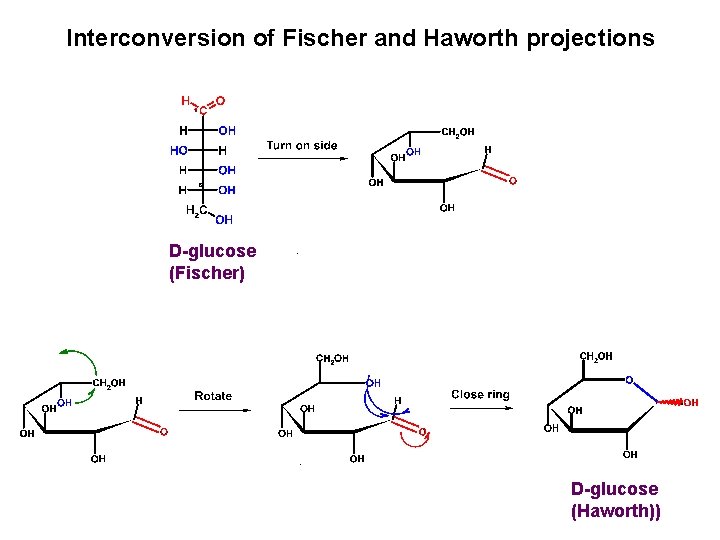
Interconversion of Fischer and Haworth projections D-glucose (Fischer) D-glucose (Haworth))
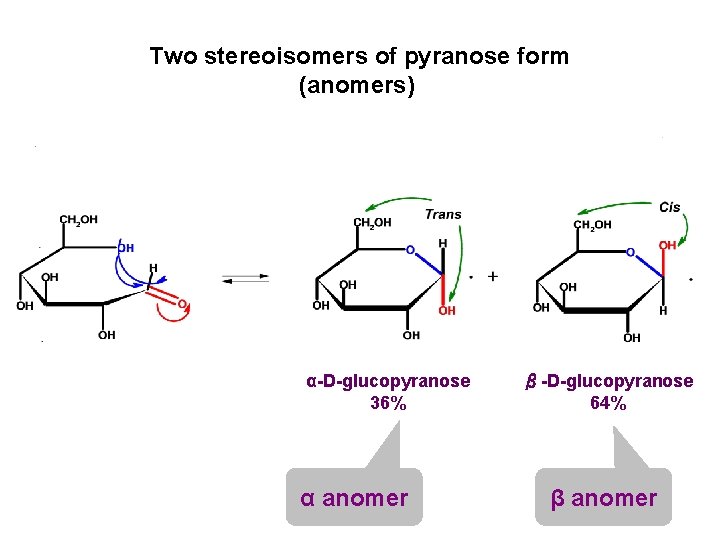
Two stereoisomers of pyranose form (anomers) α-D-glucopyranose 36% α anomer β-D-glucopyranose 64% β anomer
![Mutarotation of monosaccharides α-D-glucopyranose (36%) [α]D = +112. 2° β-D-glucopyranose (64%) [α]D = +18. Mutarotation of monosaccharides α-D-glucopyranose (36%) [α]D = +112. 2° β-D-glucopyranose (64%) [α]D = +18.](http://slidetodoc.com/presentation_image/0b747a6e679266653663969be91fc42d/image-18.jpg)
Mutarotation of monosaccharides α-D-glucopyranose (36%) [α]D = +112. 2° β-D-glucopyranose (64%) [α]D = +18. 7° At equilibrium [α]D = +52. 6°
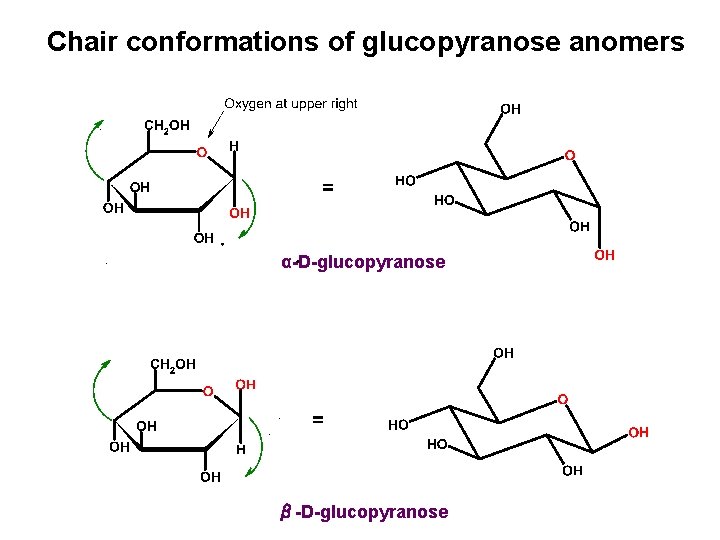
Chair conformations of glucopyranose anomers α-D-glucopyranose β-D-glucopyranose
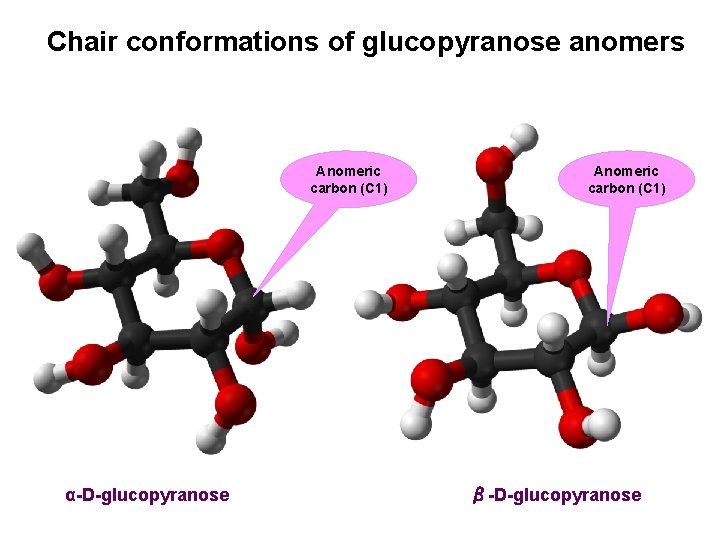
Chair conformations of glucopyranose anomers Anomeric carbon (C 1) α-D-glucopyranose Anomeric carbon (C 1) β-D-glucopyranose
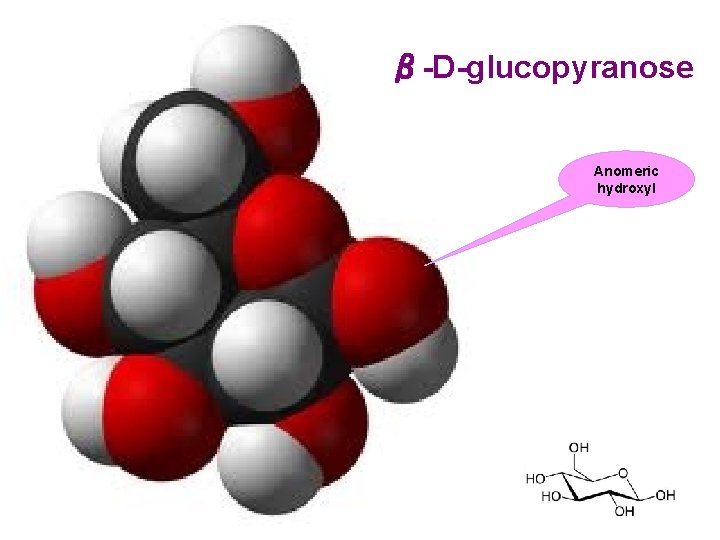
β-D-glucopyranose Anomeric hydroxyl

Physical properties of hexoses • Crystalline solids, non-volatile, decompose at elevated temperature (caramelization) • Polar, very well soluble in water, soluble to some extent in lower alcohols, insoluble in nonpolar organic solvents • Form oversaturated solutions in water (syrups) – difficult for crystallization

Chemical properties of hexoses • Enolization (isomerization) • Oxidation • Reduction • Glycoside formation (acetals) • Acylation (esters formation) • Alkylation (ethers formation) • Reactions with nitrogen nucleophiles • Kiliani-Fischer chain lengthening • Wohl degradation (chain shortening)
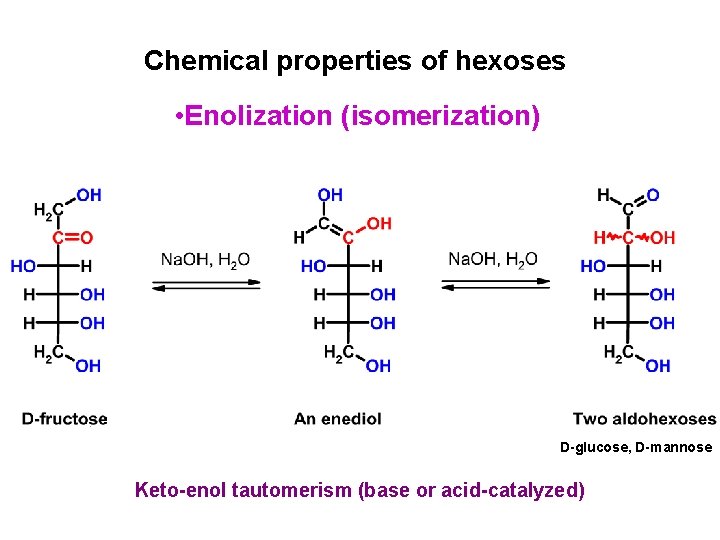
Chemical properties of hexoses • Enolization (isomerization) D-glucose, D-mannose Keto-enol tautomerism (base or acid-catalyzed)
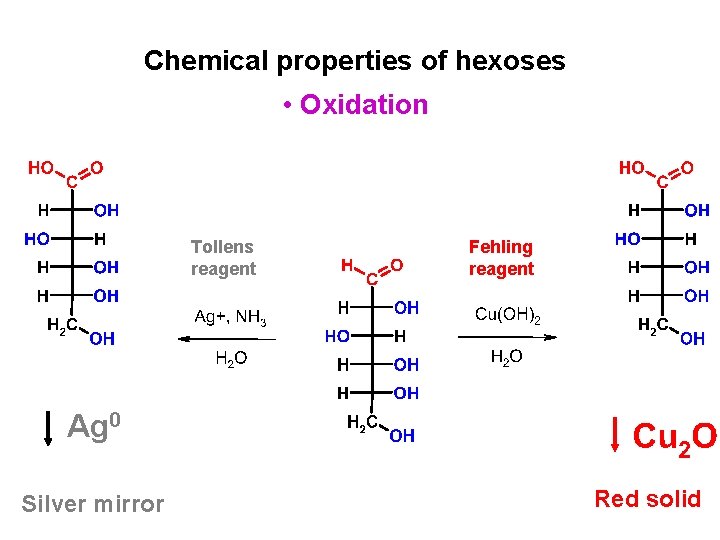
Chemical properties of hexoses • Oxidation Tollens reagent Fehling reagent Ag 0 Silver mirror Red solid
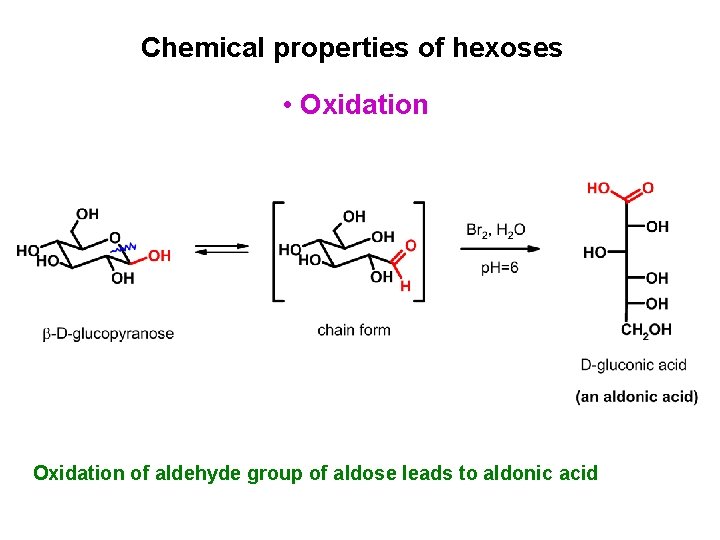
Chemical properties of hexoses • Oxidation of aldehyde group of aldose leads to aldonic acid
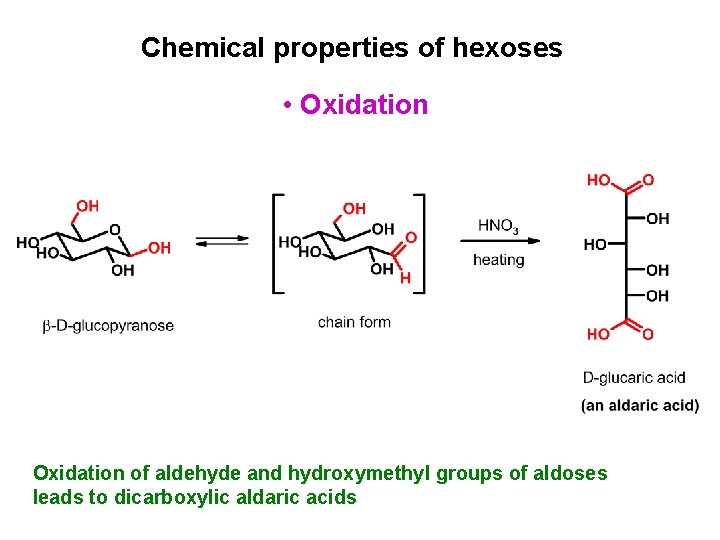
Chemical properties of hexoses • Oxidation of aldehyde and hydroxymethyl groups of aldoses leads to dicarboxylic aldaric acids
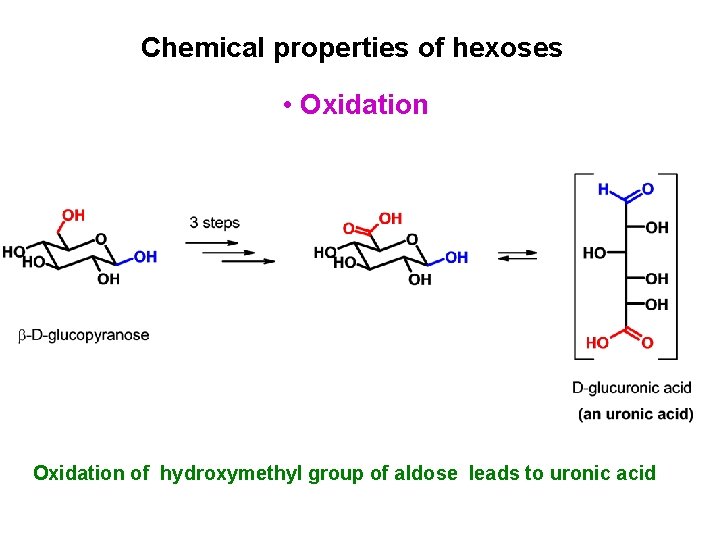
Chemical properties of hexoses • Oxidation of hydroxymethyl group of aldose leads to uronic acid
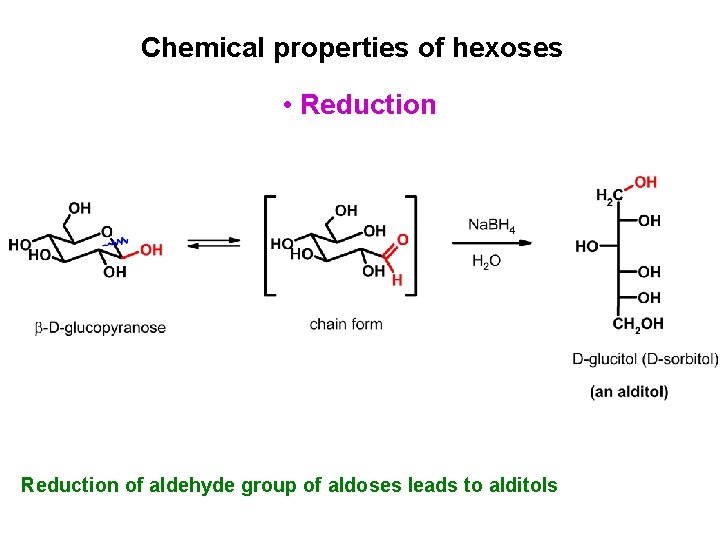
Chemical properties of hexoses • Reduction of aldehyde group of aldoses leads to alditols
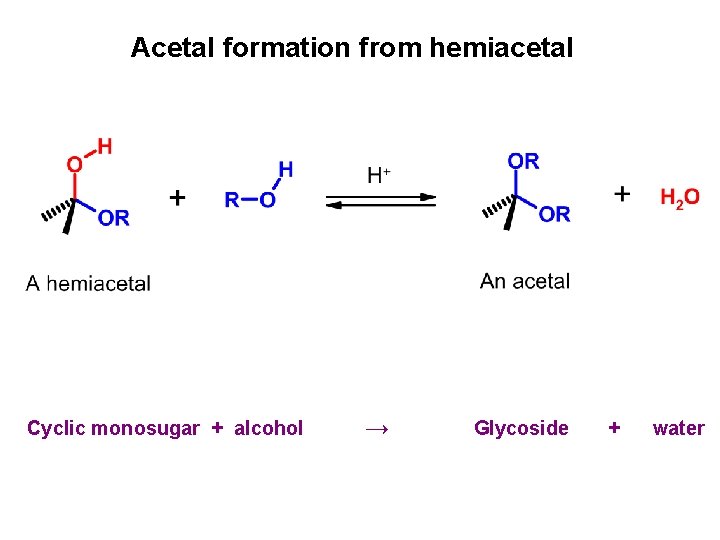
Acetal formation from hemiacetal Cyclic monosugar + alcohol → Glycoside + water
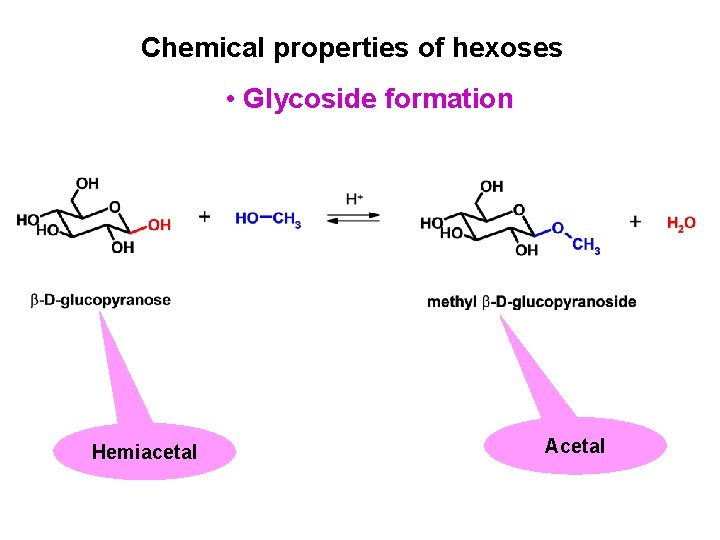
Chemical properties of hexoses • Glycoside formation Hemiacetal Acetal
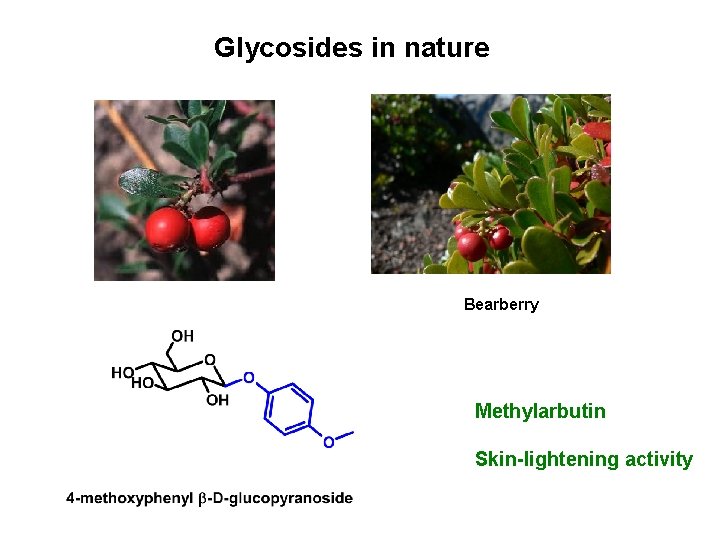
Glycosides in nature Bearberry Methylarbutin Skin-lightening activity
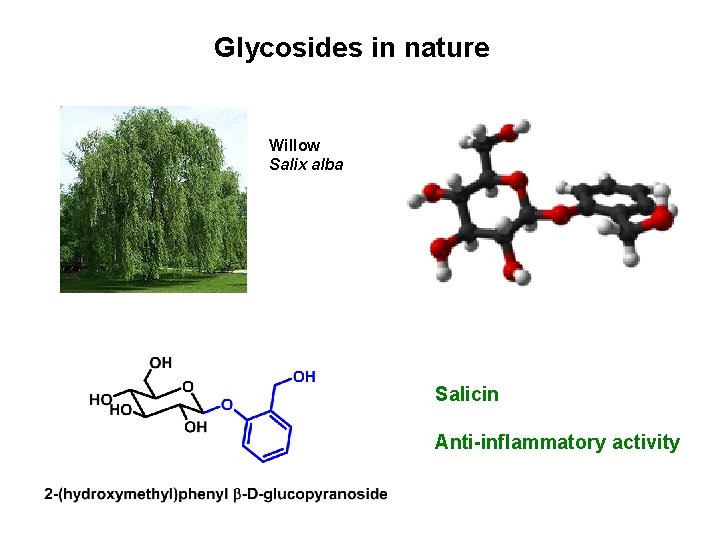
Glycosides in nature Willow Salix alba Salicin Anti-inflammatory activity
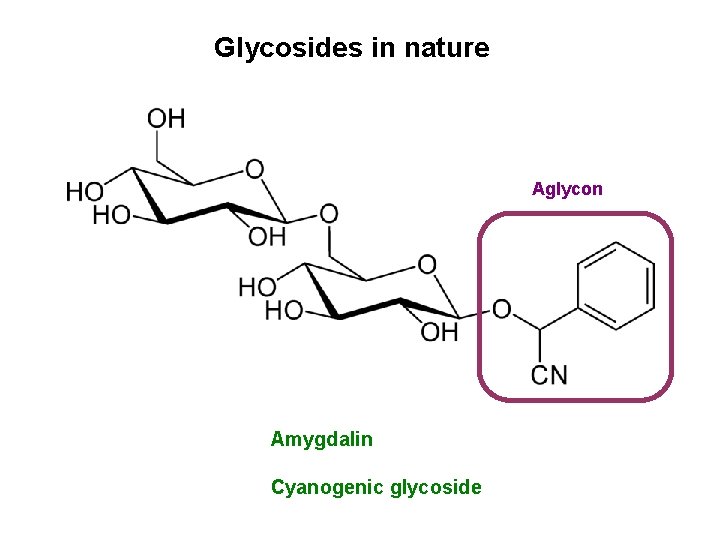
Glycosides in nature Aglycon Amygdalin Cyanogenic glycoside

Properties of glycosides • Exist as two distinct anomers - α or β • Do not show reducing properties (ring does not open) • Mutarotation is not possible (ring does not open) • Stable in alkaline aq. solutions (like ethers) • Hydrolyze in acidic aq. solutions into sugar and aglycon
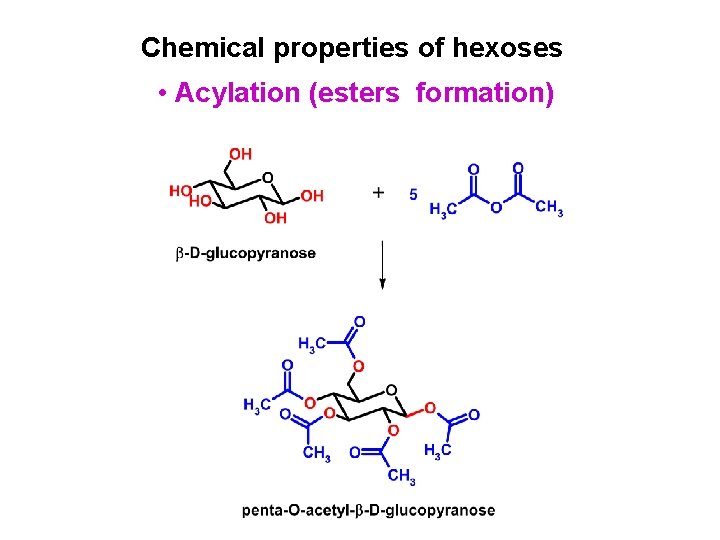
Chemical properties of hexoses • Acylation (esters formation)
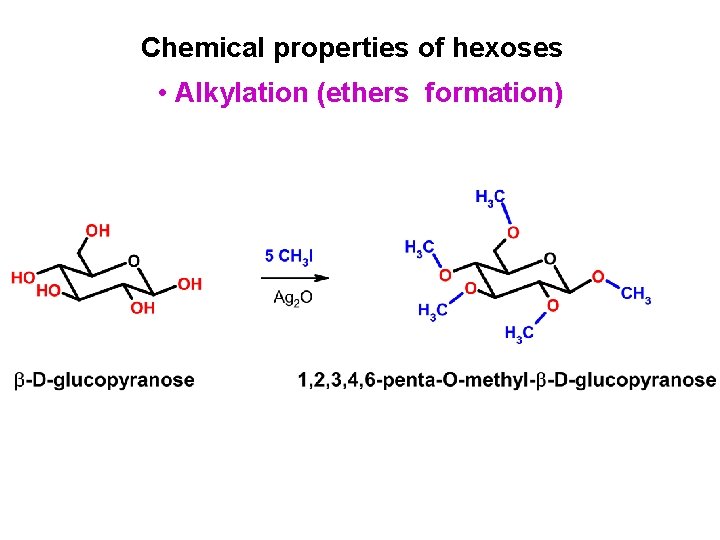
Chemical properties of hexoses • Alkylation (ethers formation)
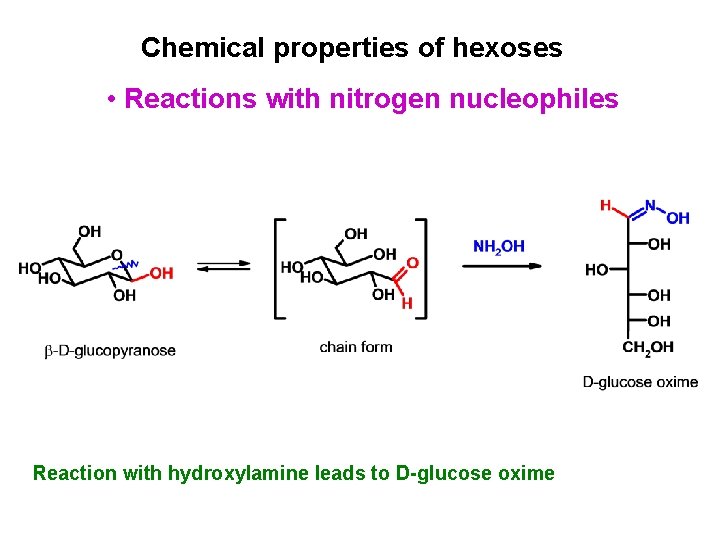
Chemical properties of hexoses • Reactions with nitrogen nucleophiles Reaction with hydroxylamine leads to D-glucose oxime
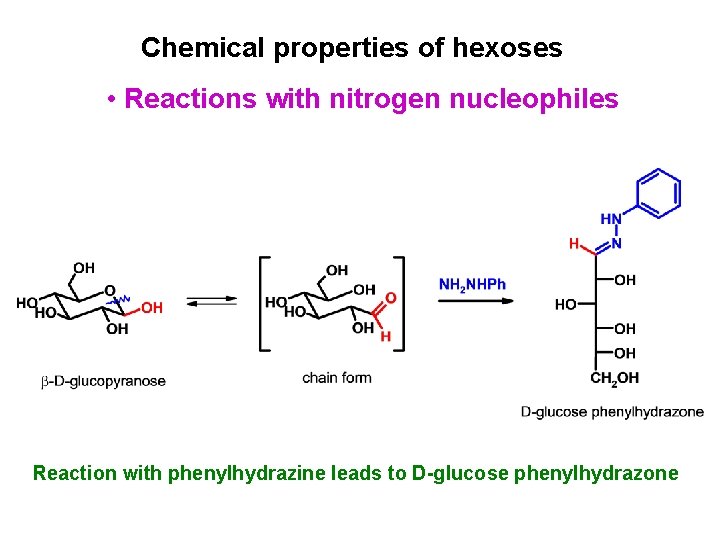
Chemical properties of hexoses • Reactions with nitrogen nucleophiles Reaction with phenylhydrazine leads to D-glucose phenylhydrazone
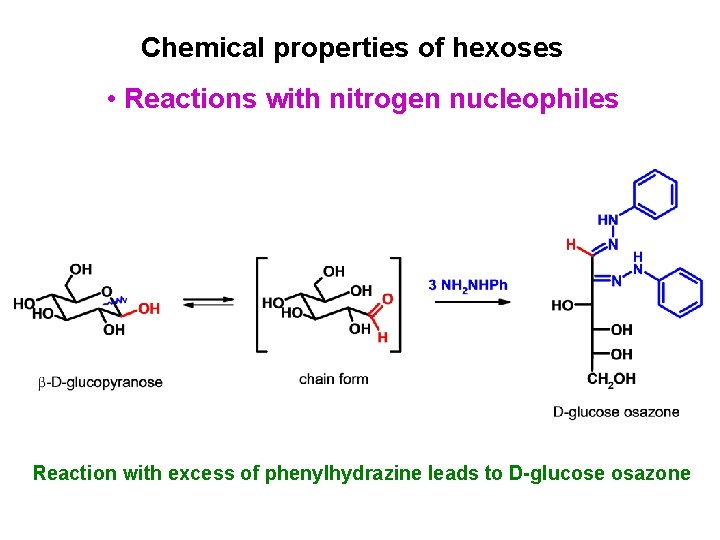
Chemical properties of hexoses • Reactions with nitrogen nucleophiles Reaction with excess of phenylhydrazine leads to D-glucose osazone
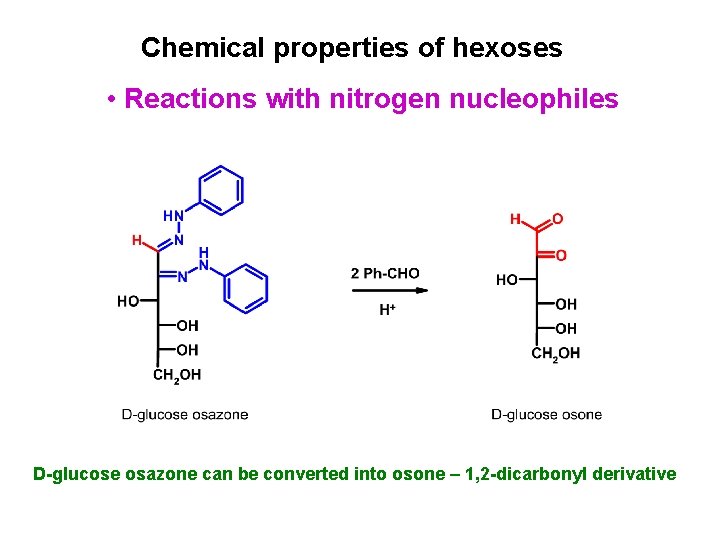
Chemical properties of hexoses • Reactions with nitrogen nucleophiles D-glucose osazone can be converted into osone – 1, 2 -dicarbonyl derivative
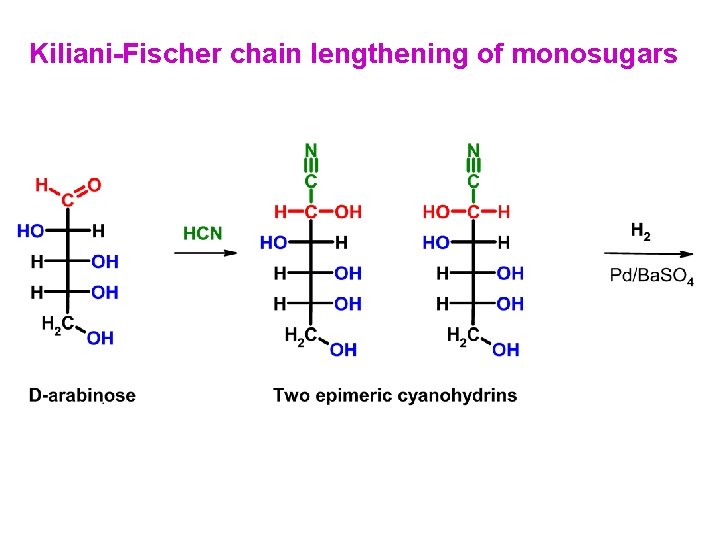
Kiliani-Fischer chain lengthening of monosugars
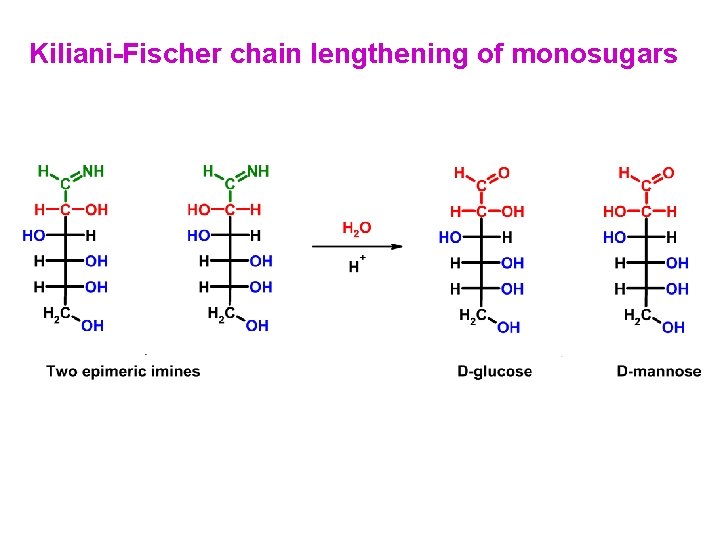
Kiliani-Fischer chain lengthening of monosugars
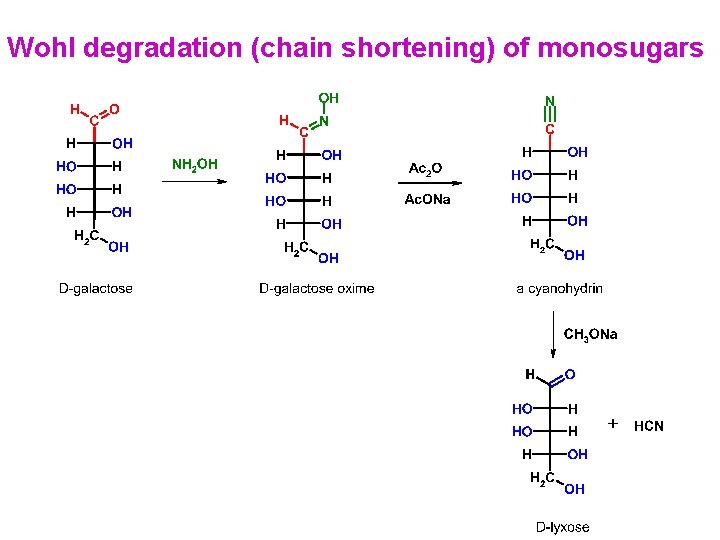
Wohl degradation (chain shortening) of monosugars
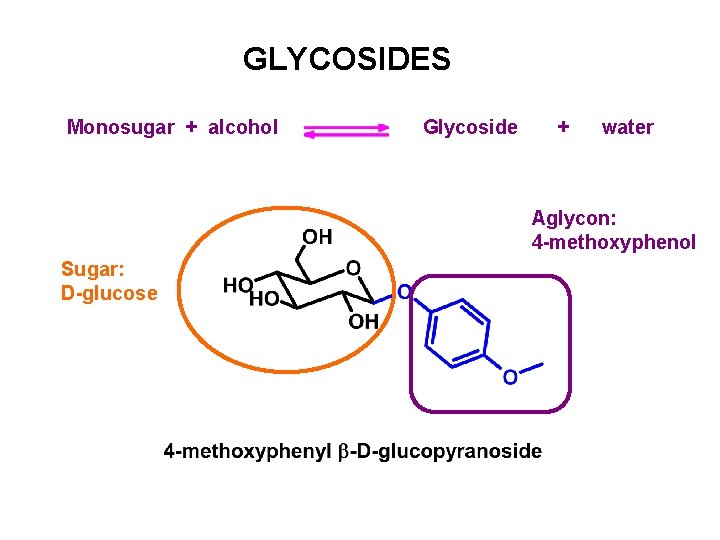
GLYCOSIDES Monosugar + alcohol Glycoside + water Aglycon: 4 -methoxyphenol Sugar: D-glucose
OLIGOSACCHARIDES
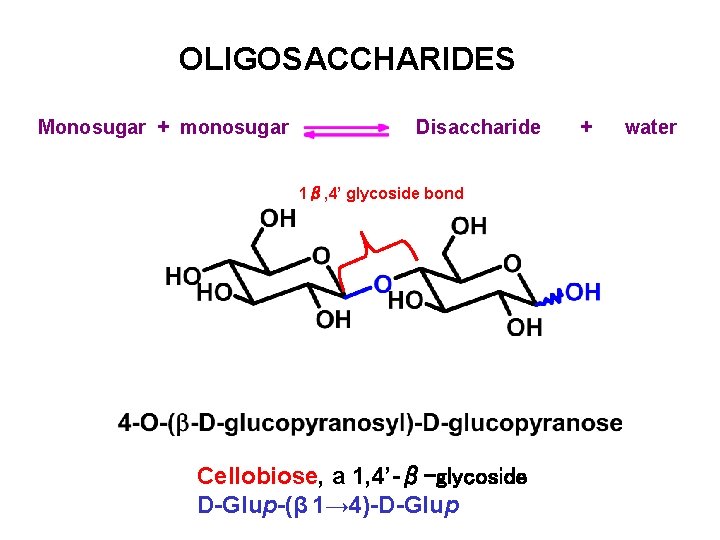
OLIGOSACCHARIDES Monosugar + monosugar Disaccharide 1β, 4’ glycoside bond Cellobiose, a 1, 4’-β-glycoside D-Glup-(β 1→ 4)-D-Glup + water
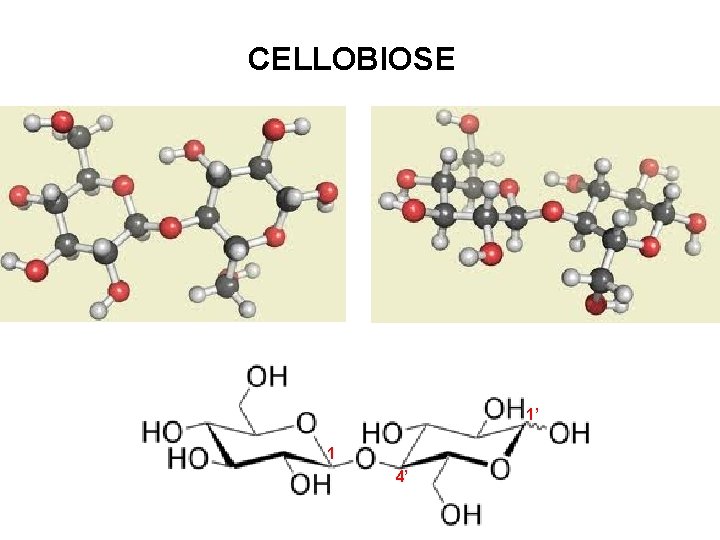
CELLOBIOSE 1’ 1 4’
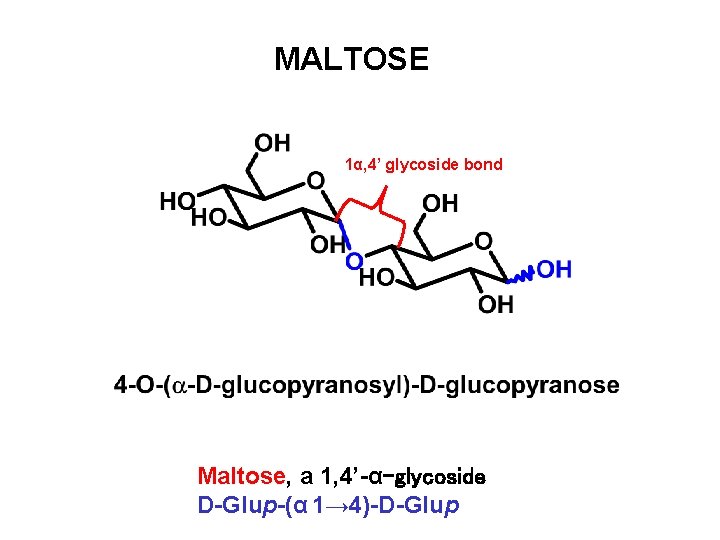
MALTOSE 1α, 4’ glycoside bond Maltose, a 1, 4’-α-glycoside D-Glup-(α 1→ 4)-D-Glup
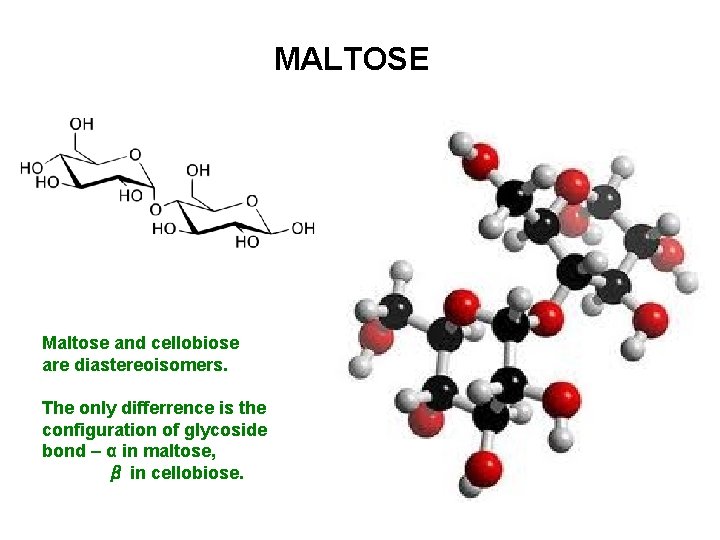
MALTOSE Maltose and cellobiose are diastereoisomers. The only differrence is the configuration of glycoside bond – α in maltose, β in cellobiose.
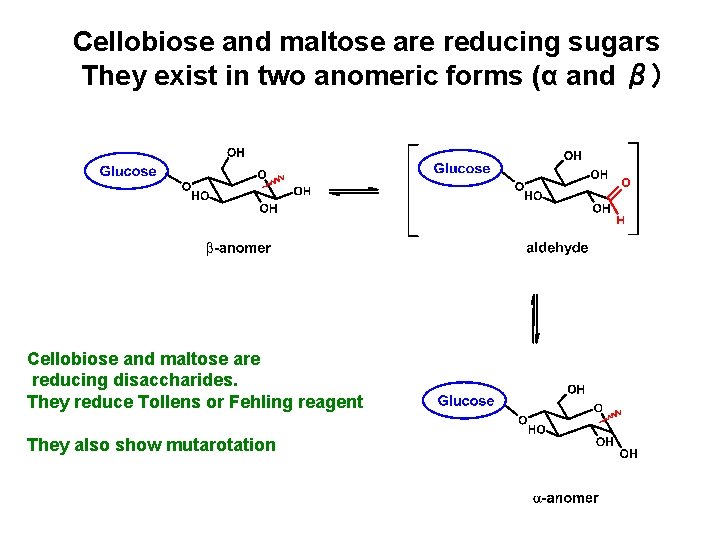
Cellobiose and maltose are reducing sugars They exist in two anomeric forms (α and β) Cellobiose and maltose are reducing disaccharides. They reduce Tollens or Fehling reagent They also show mutarotation
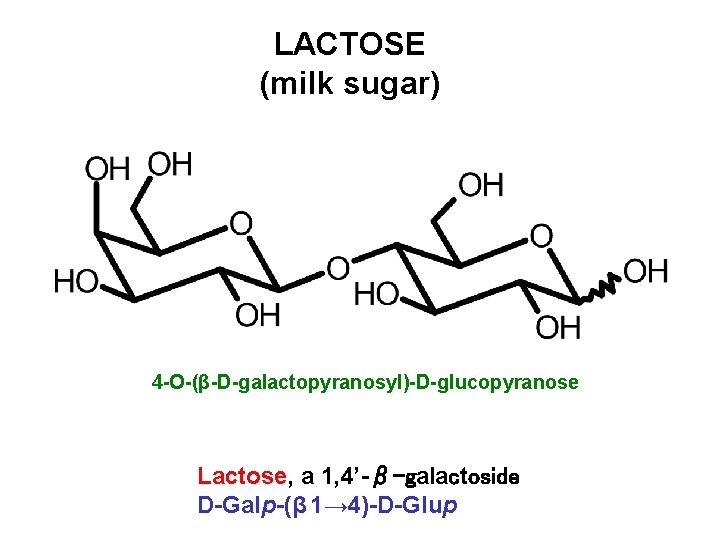
LACTOSE (milk sugar) 4 -O-(β-D-galactopyranosyl)-D-glucopyranose Lactose, a 1, 4’-β-galactoside D-Galp-(β 1→ 4)-D-Glup
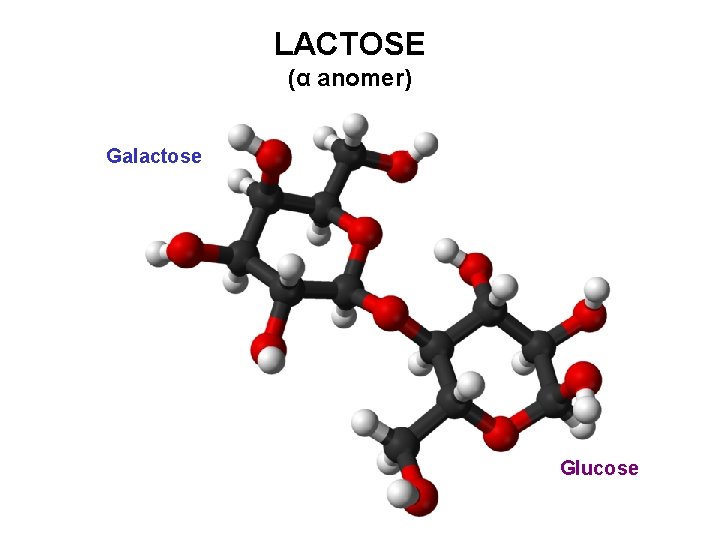
LACTOSE (α anomer) Galactose Glucose
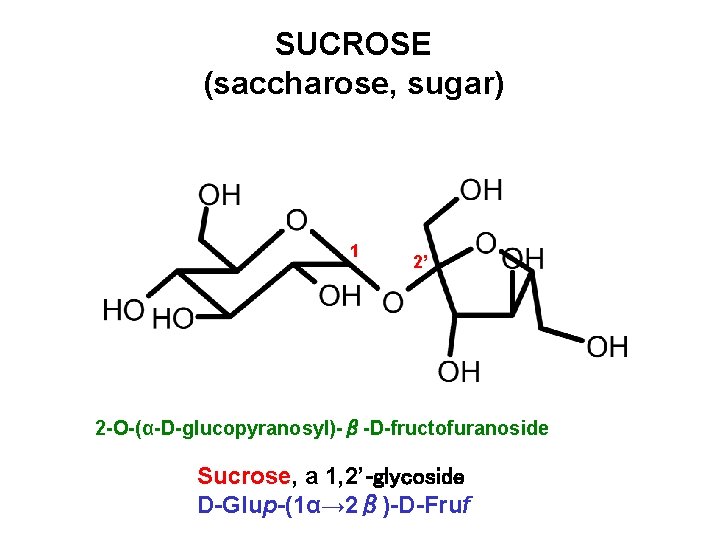
SUCROSE (saccharose, sugar) 1 2’ 2 -O-(α-D-glucopyranosyl)-β-D-fructofuranoside Sucrose, a 1, 2’-glycoside D-Glup-(1α→ 2β)-D-Fruf
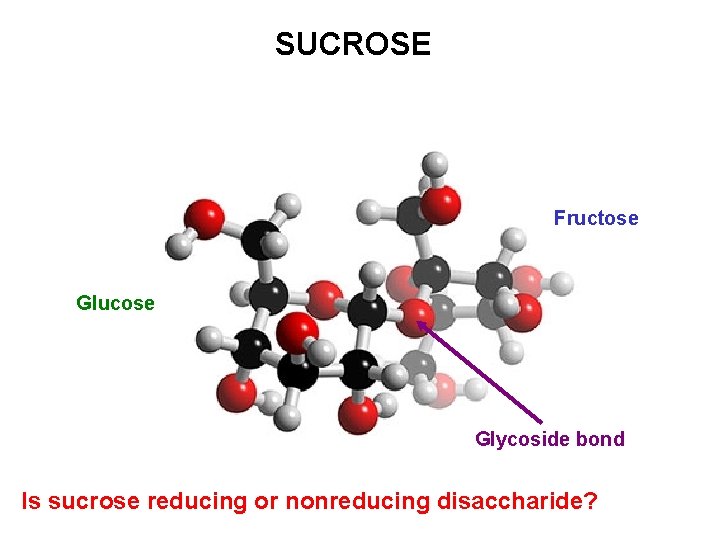
SUCROSE Fructose Glucose Glycoside bond Is sucrose reducing or nonreducing disaccharide?
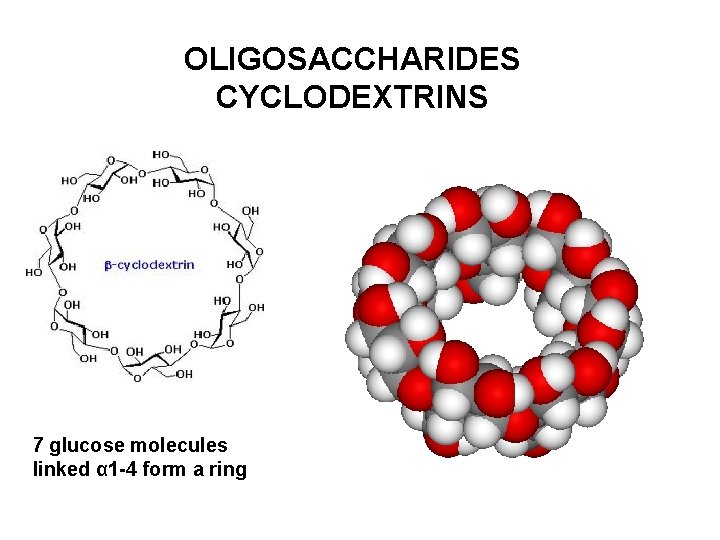
OLIGOSACCHARIDES CYCLODEXTRINS 7 glucose molecules linked α 1 -4 form a ring
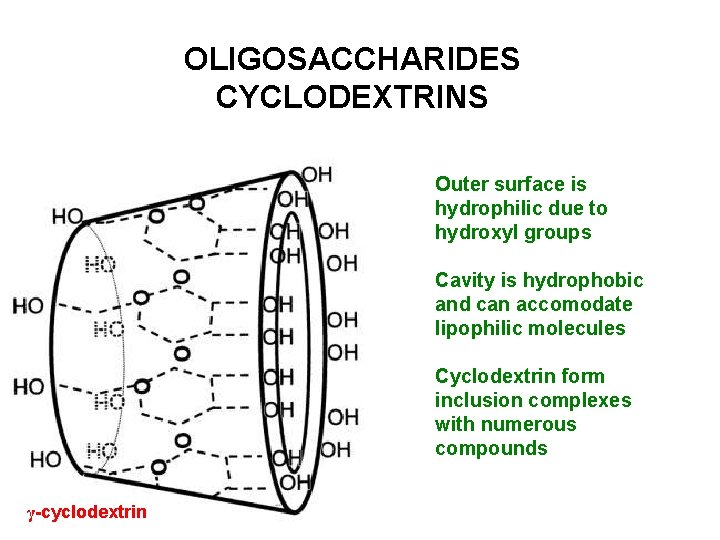
OLIGOSACCHARIDES CYCLODEXTRINS Outer surface is hydrophilic due to hydroxyl groups Cavity is hydrophobic and can accomodate lipophilic molecules Cyclodextrin form inclusion complexes with numerous compounds γ-cyclodextrin
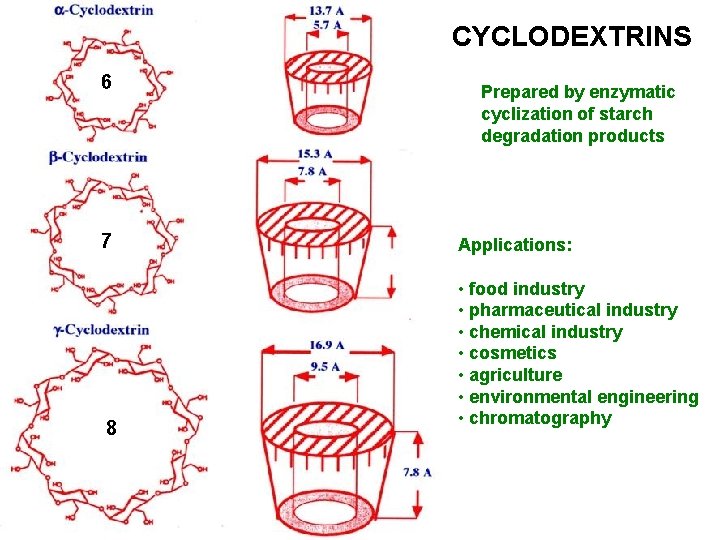
CYCLODEXTRINS 6 7 8 Prepared by enzymatic cyclization of starch degradation products Applications: • food industry • pharmaceutical industry • chemical industry • cosmetics • agriculture • environmental engineering • chromatography

POLYSACCHARIDES
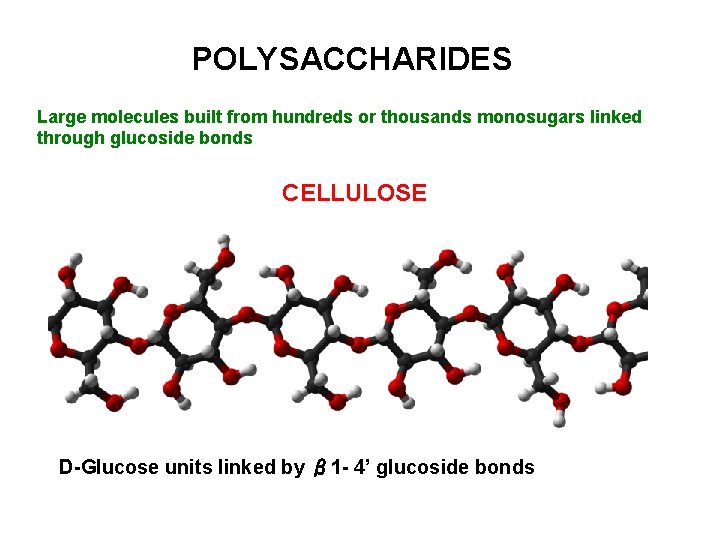
POLYSACCHARIDES Large molecules built from hundreds or thousands monosugars linked through glucoside bonds CELLULOSE D-Glucose units linked by β 1 - 4’ glucoside bonds
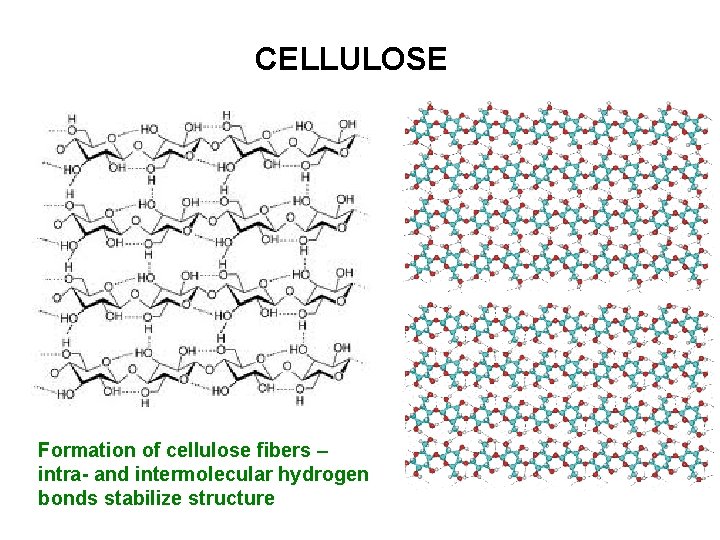
CELLULOSE Formation of cellulose fibers – intra- and intermolecular hydrogen bonds stabilize structure
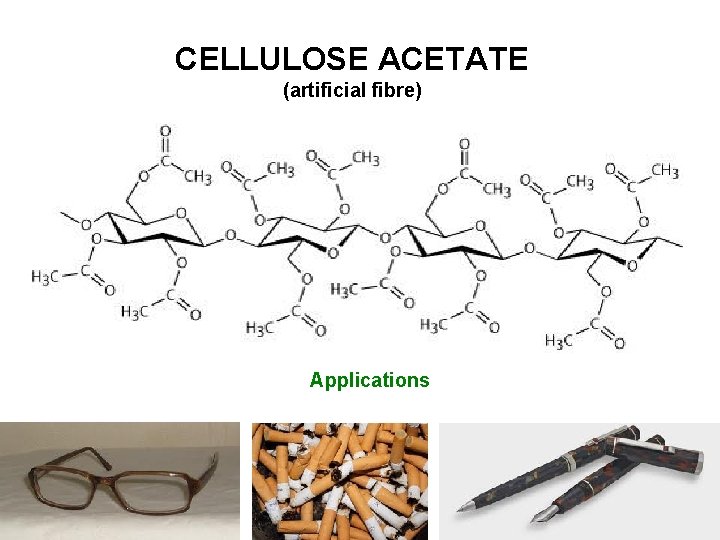
CELLULOSE ACETATE (artificial fibre) Applications
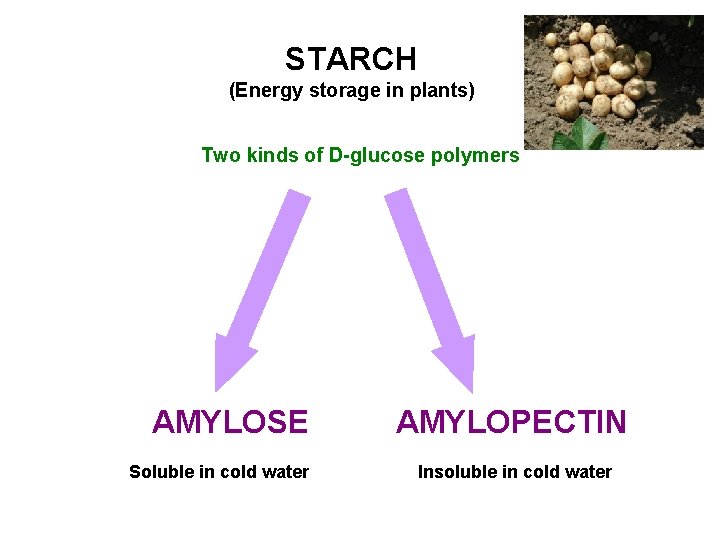
STARCH (Energy storage in plants) Two kinds of D-glucose polymers AMYLOSE Soluble in cold water AMYLOPECTIN Insoluble in cold water
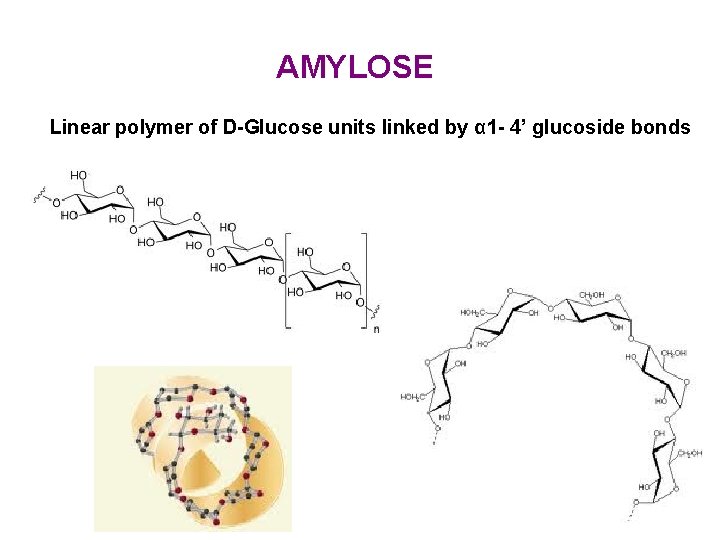
AMYLOSE Linear polymer of D-Glucose units linked by α 1 - 4’ glucoside bonds
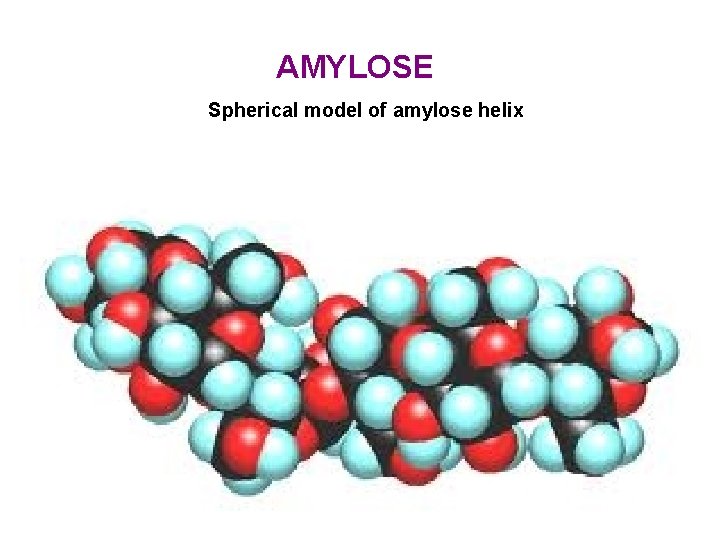
AMYLOSE Spherical model of amylose helix
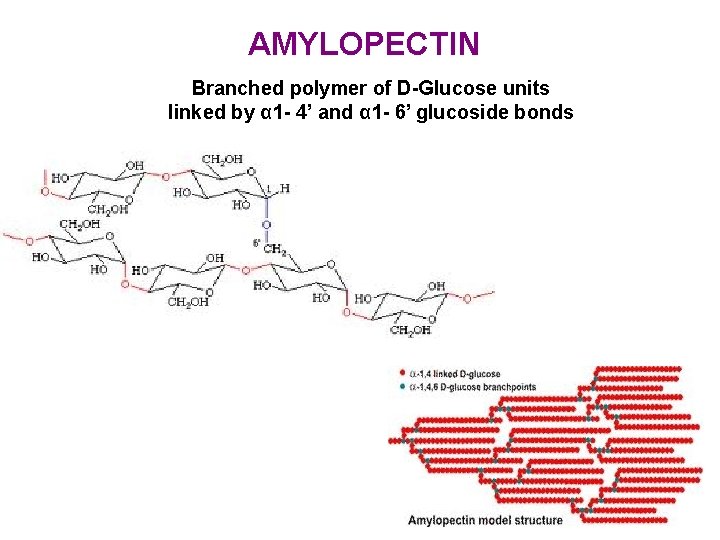
AMYLOPECTIN Branched polymer of D-Glucose units linked by α 1 - 4’ and α 1 - 6’ glucoside bonds
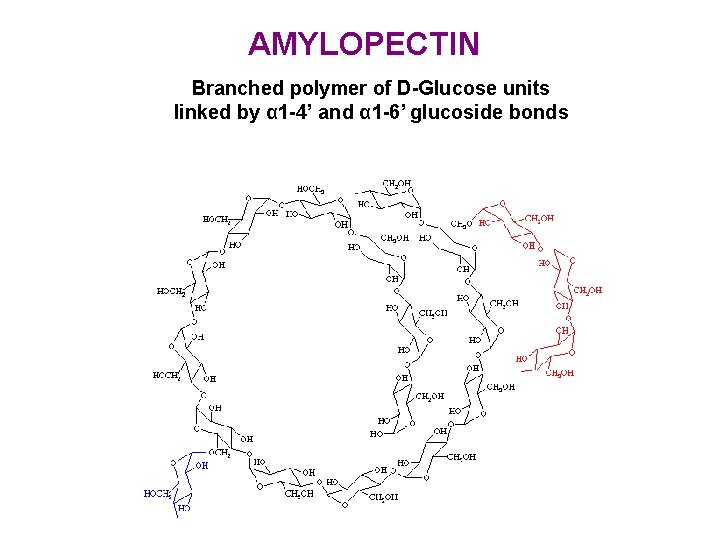
AMYLOPECTIN Branched polymer of D-Glucose units linked by α 1 -4’ and α 1 -6’ glucoside bonds
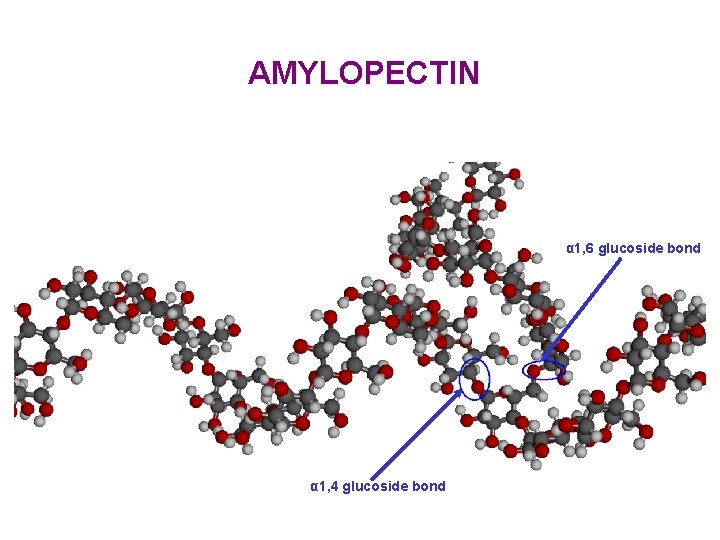
AMYLOPECTIN α 1, 6 glucoside bond α 1, 4 glucoside bond

GLYCOGEN Branched polymer of D-Glucose units linked by α 1 - 4’ and α 1 - 6’ glucoside bonds
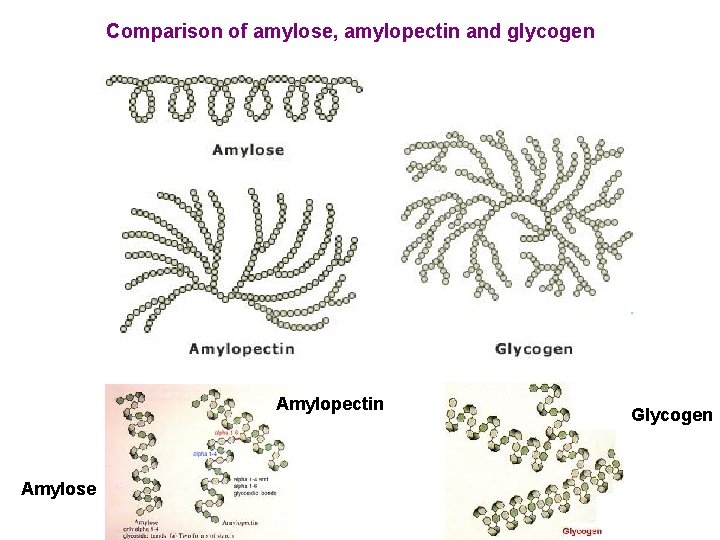
Comparison of amylose, amylopectin and glycogen Amylopectin Amylose Glycogen
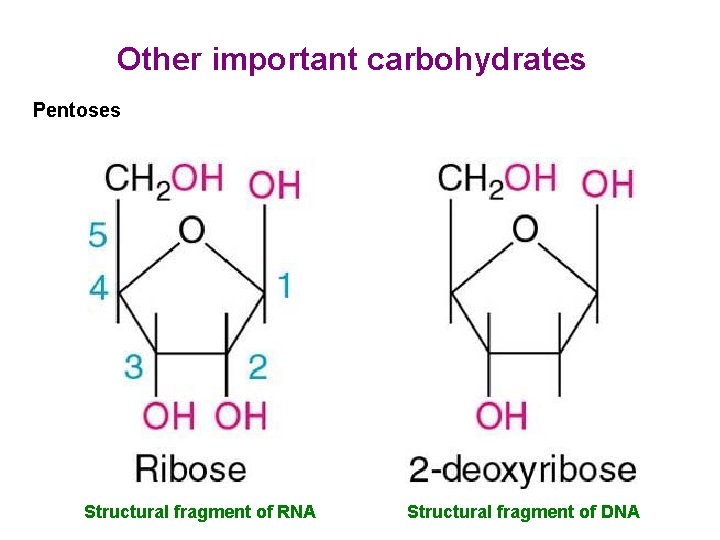
Other important carbohydrates Pentoses Structural fragment of RNA Structural fragment of DNA
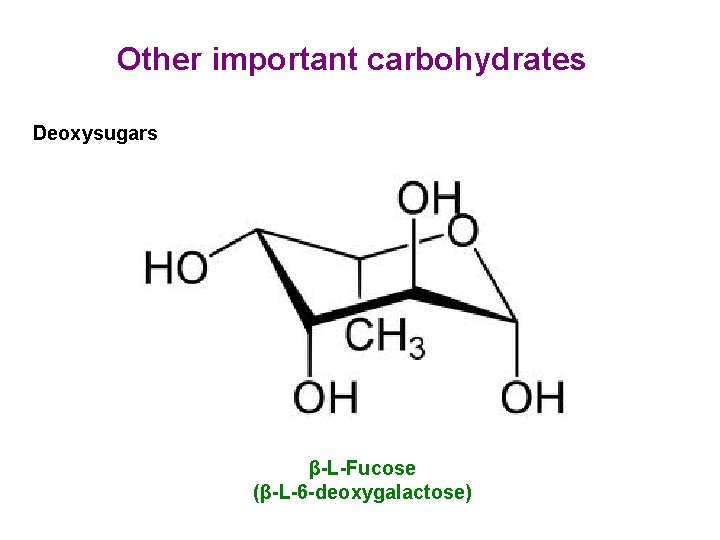
Other important carbohydrates Deoxysugars β-L-Fucose (β-L-6 -deoxygalactose)
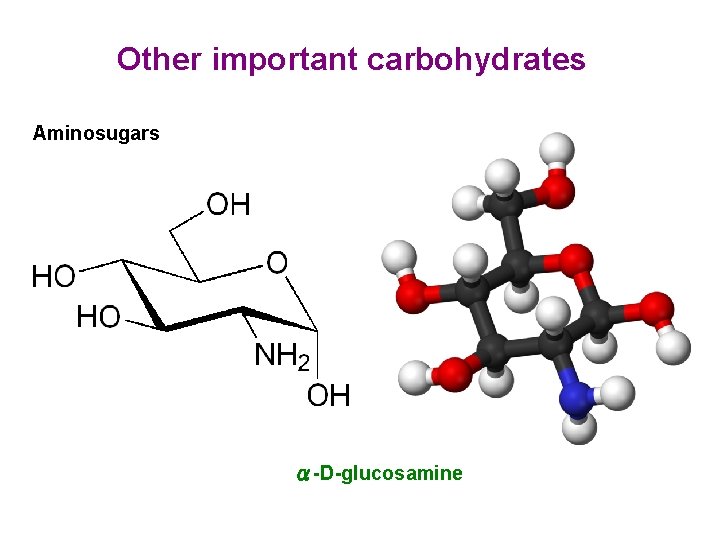
Other important carbohydrates Aminosugars α-D-glucosamine
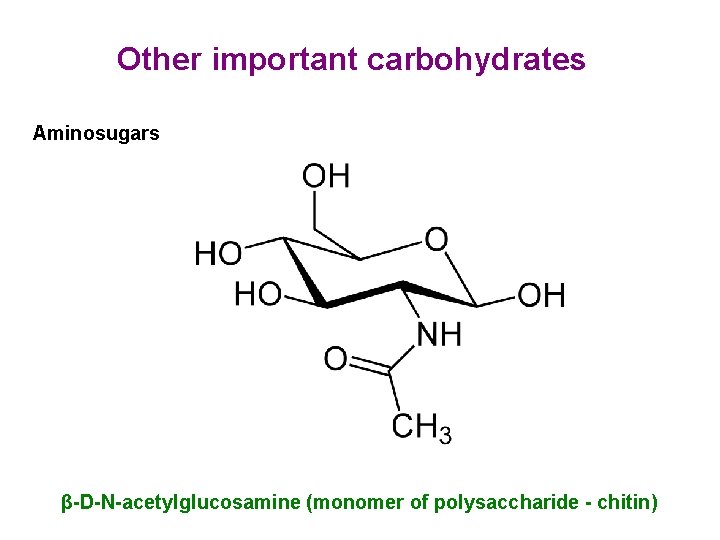
Other important carbohydrates Aminosugars β-D-N-acetylglucosamine (monomer of polysaccharide - chitin)
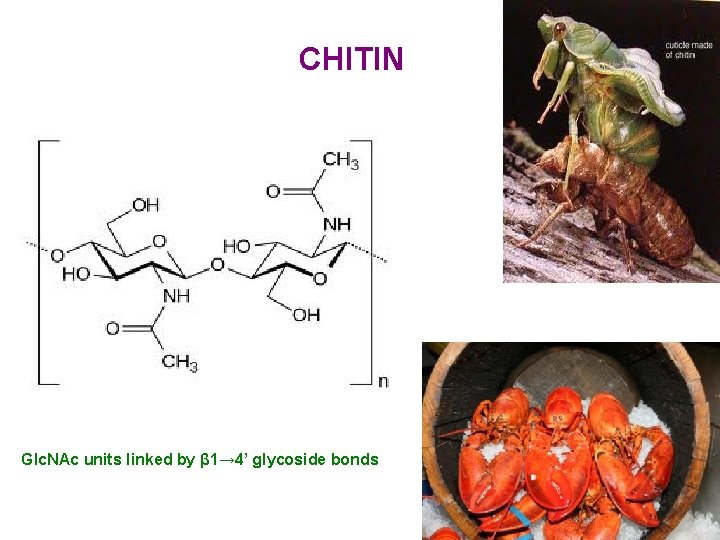
CHITIN Glc. NAc units linked by β 1→ 4’ glycoside bonds
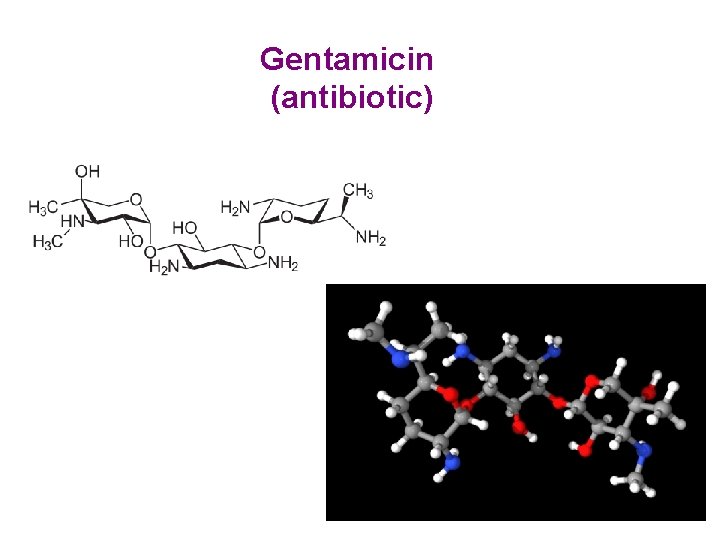
Gentamicin (antibiotic)
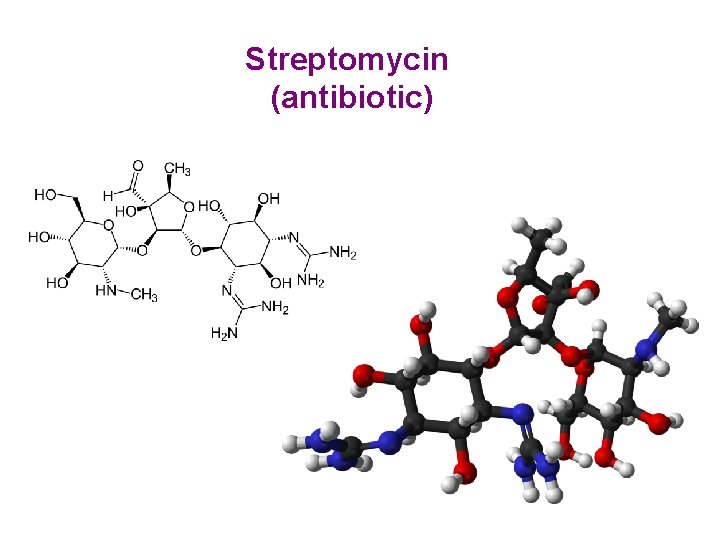
Streptomycin (antibiotic)
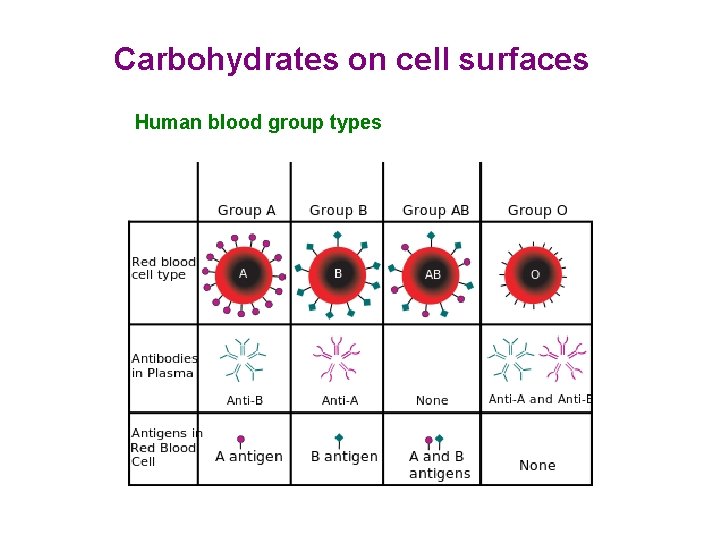
Carbohydrates on cell surfaces Human blood group types
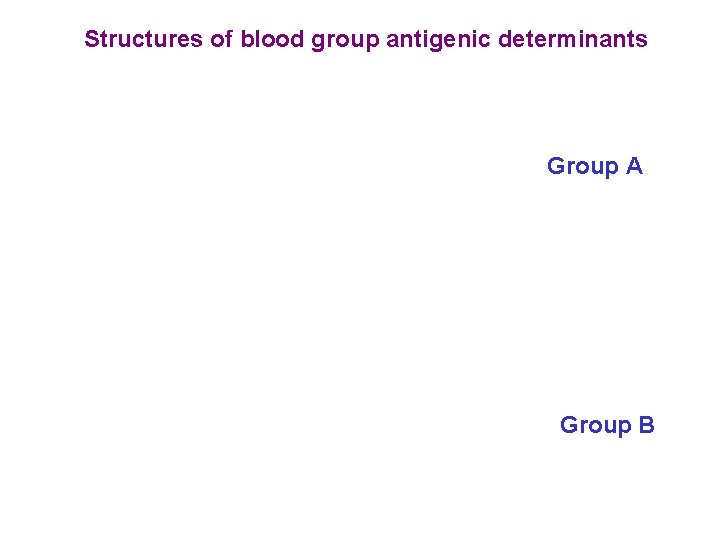
Structures of blood group antigenic determinants Group A Group B
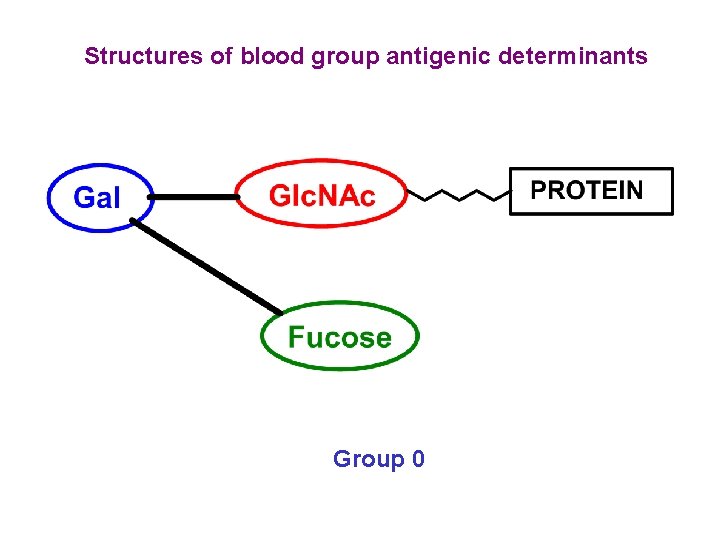
Structures of blood group antigenic determinants Group 0
![Red blood cell compatibility Recipient[1] O− O+ A− A+ B− B+ AB− AB+ Donor[1] Red blood cell compatibility Recipient[1] O− O+ A− A+ B− B+ AB− AB+ Donor[1]](http://slidetodoc.com/presentation_image/0b747a6e679266653663969be91fc42d/image-81.jpg)
Red blood cell compatibility Recipient[1] O− O+ A− A+ B− B+ AB− AB+ Donor[1] O- O+ A− A+ B− B+ AB− AB+ compatible incompatible
- Slides: 81