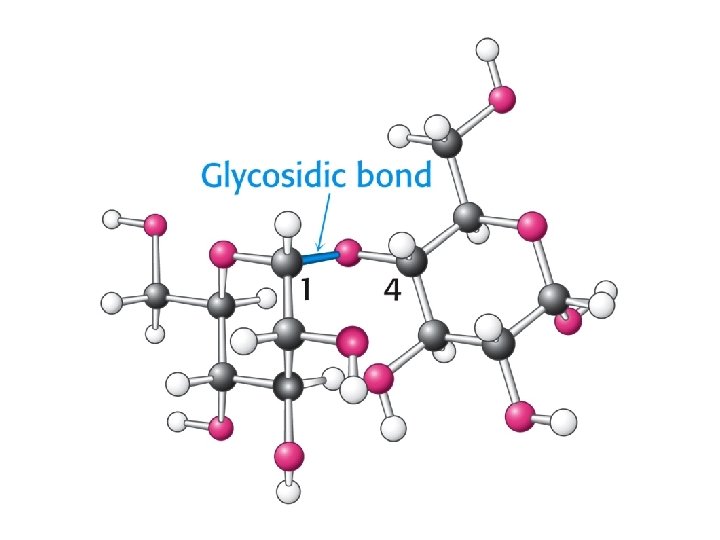
Carbohydrates Reducing sugars Modified monosaccharides Glycosides Disaccharides Glycogen

Carbohydrates
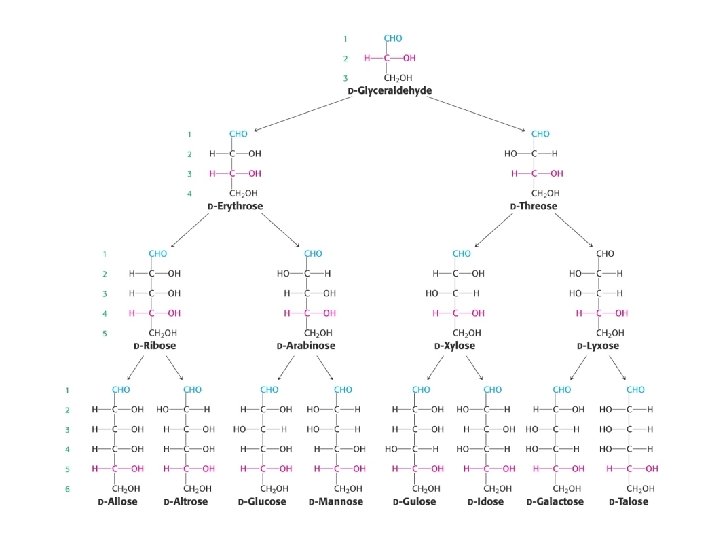
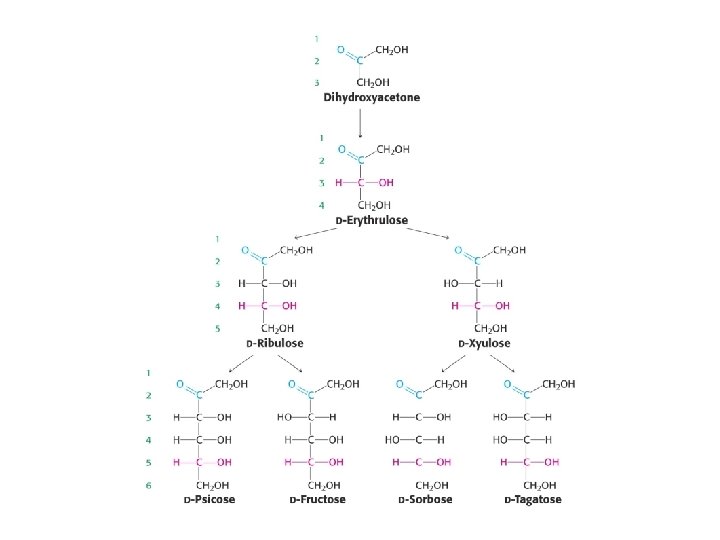
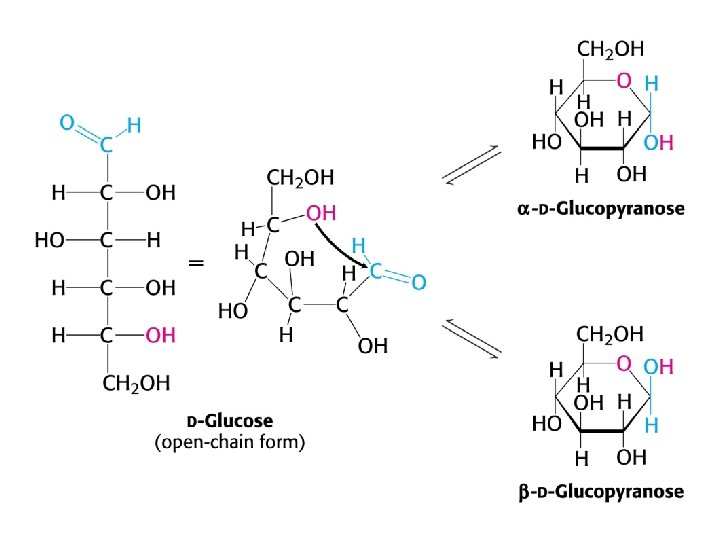
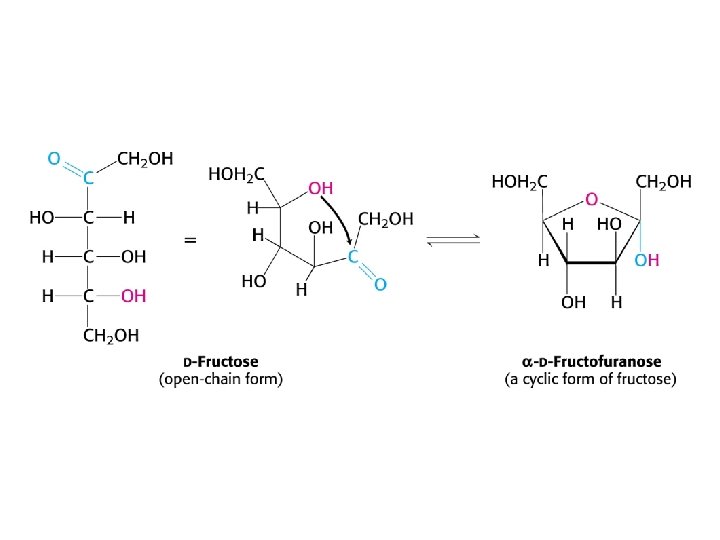
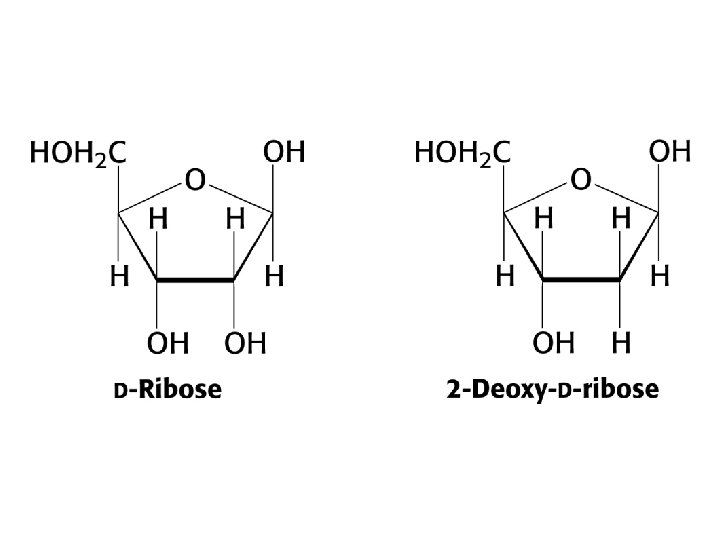
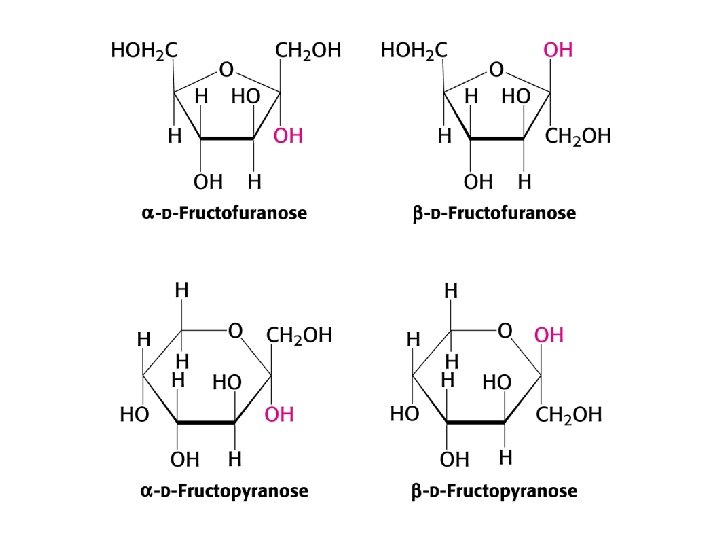
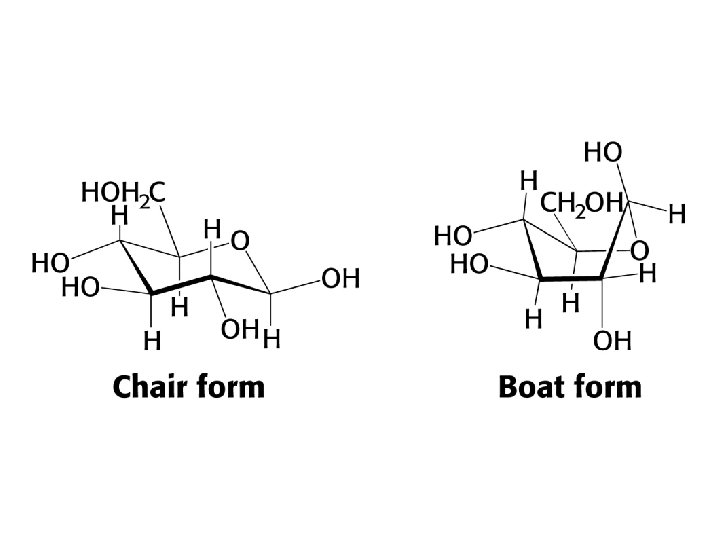
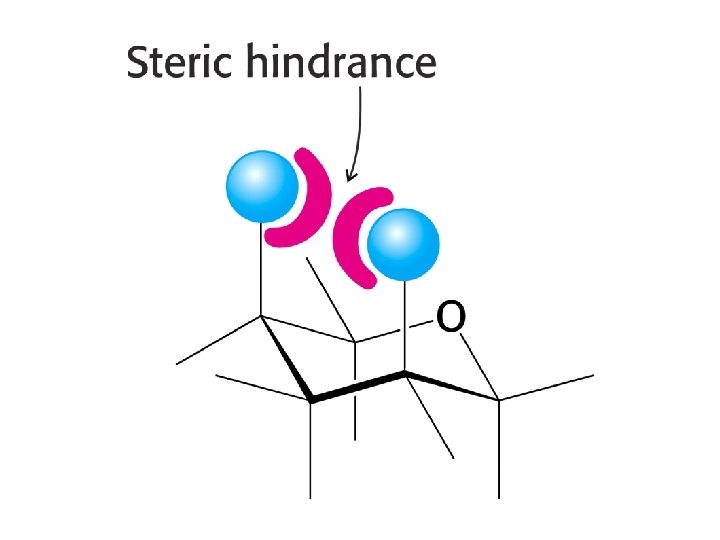
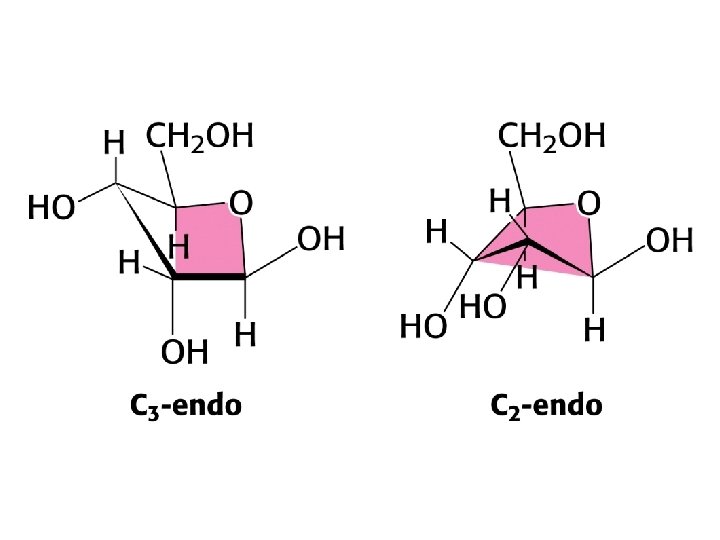
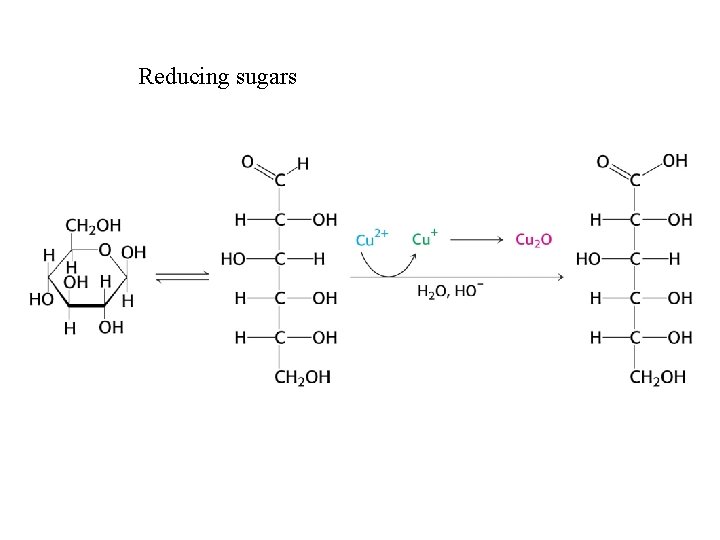
Reducing sugars
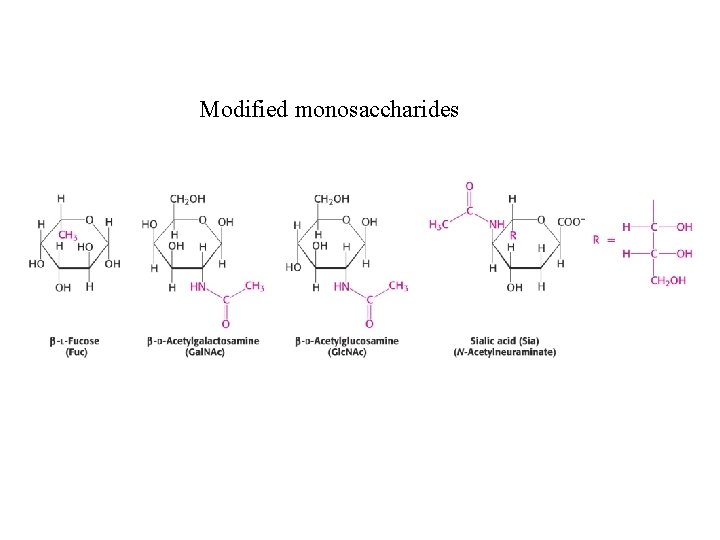
Modified monosaccharides
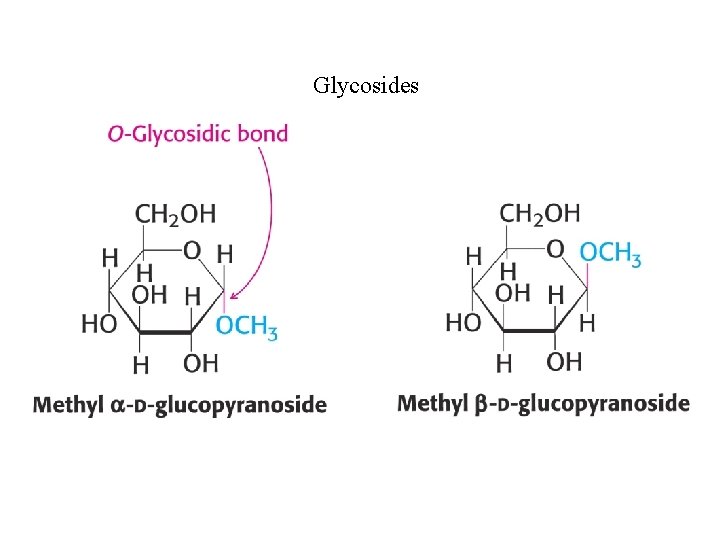
Glycosides
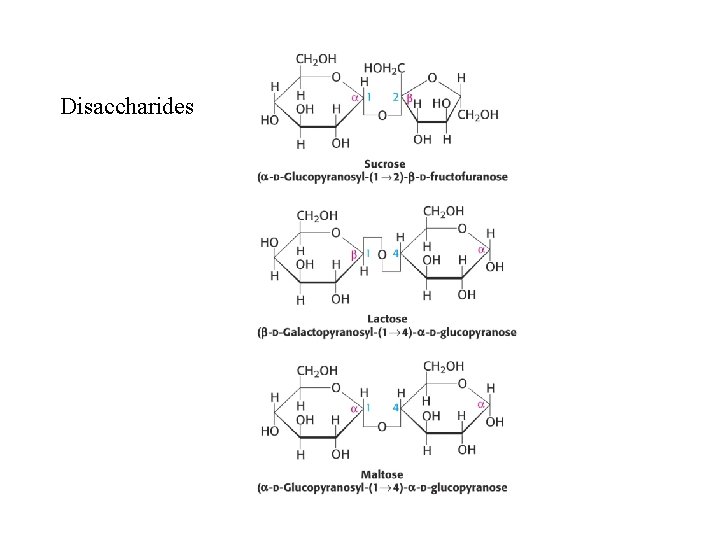
Disaccharides
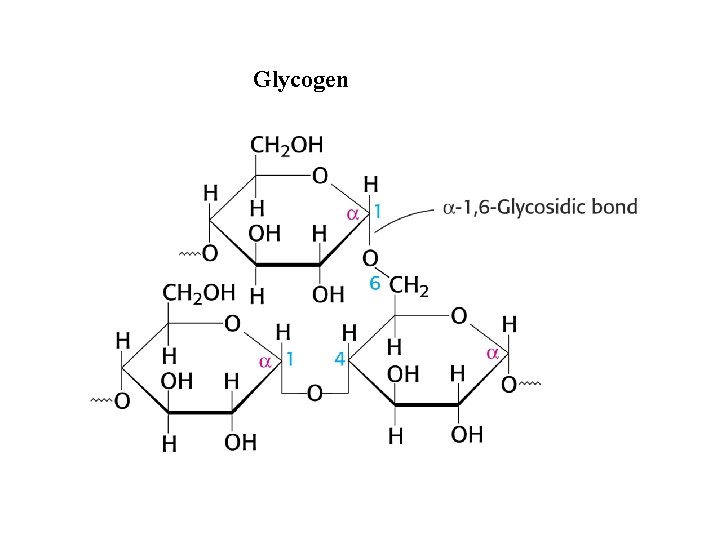
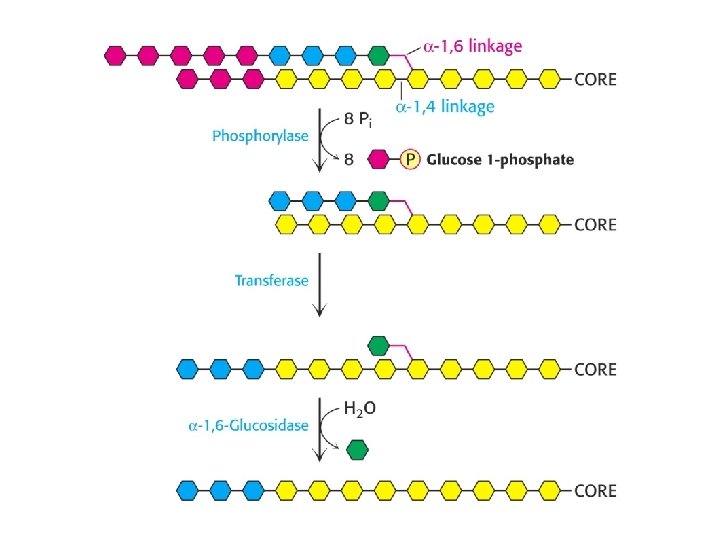
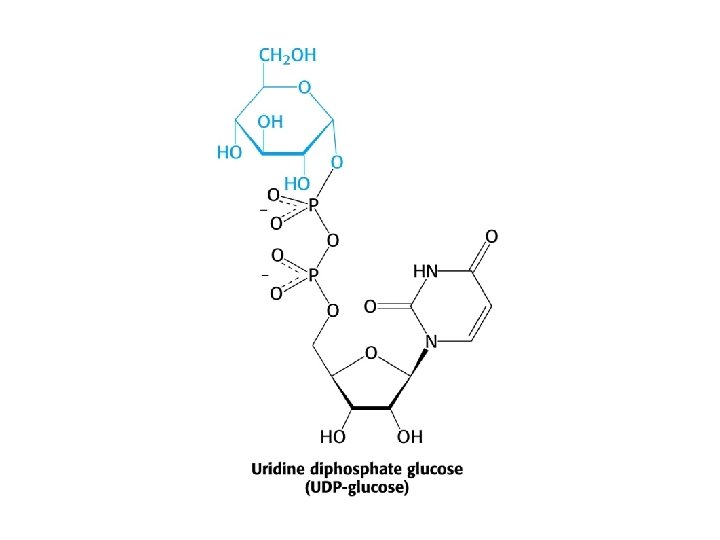
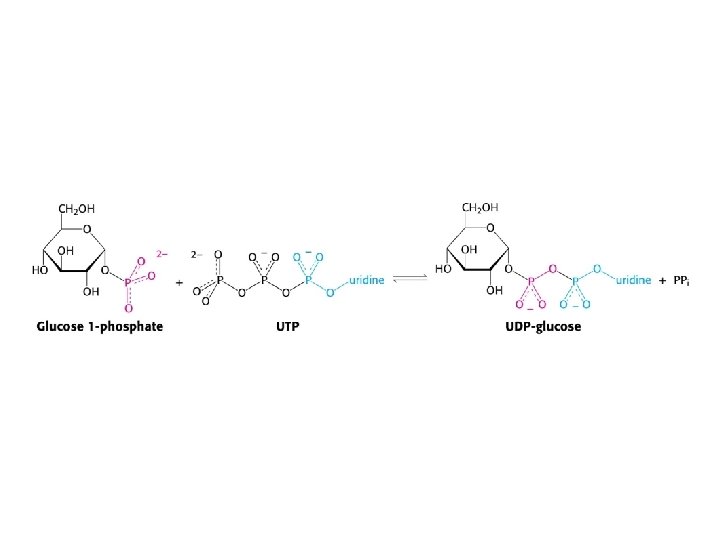
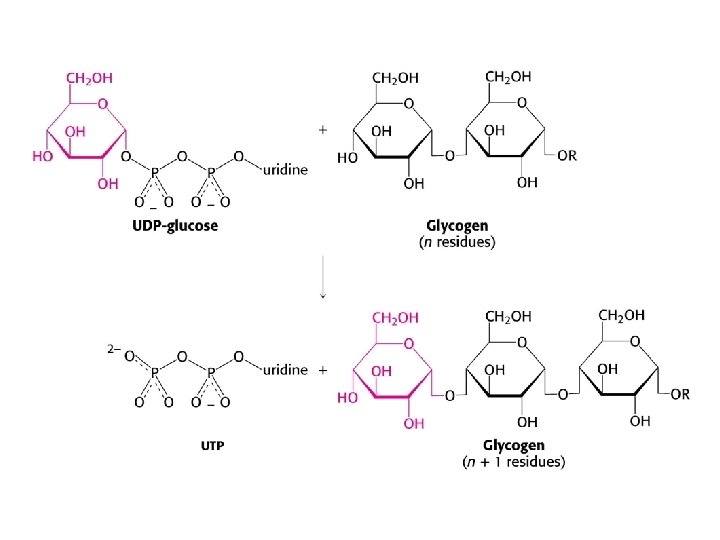
Glycogen
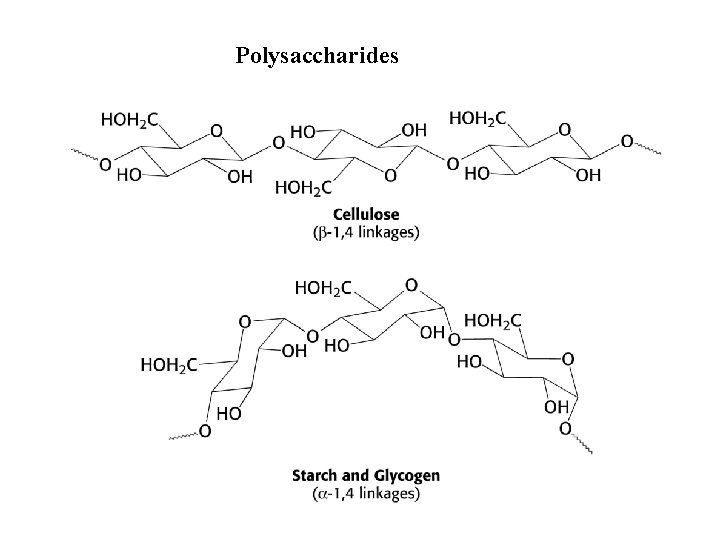
Polysaccharides
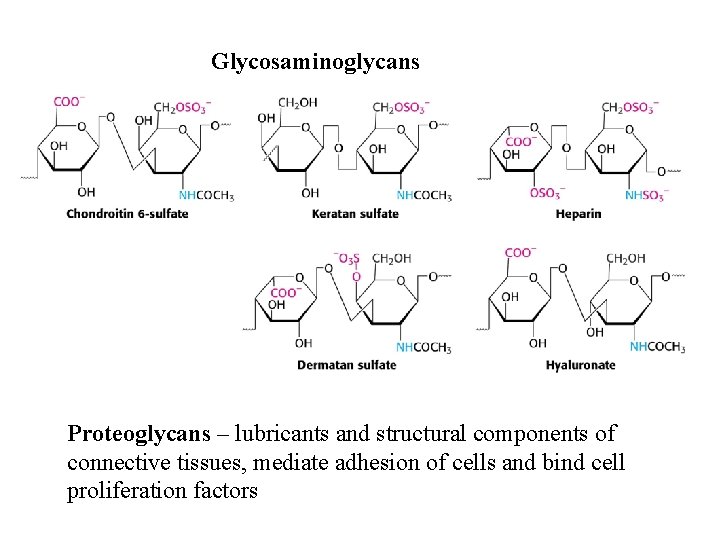
Glycosaminoglycans Proteoglycans – lubricants and structural components of connective tissues, mediate adhesion of cells and bind cell proliferation factors
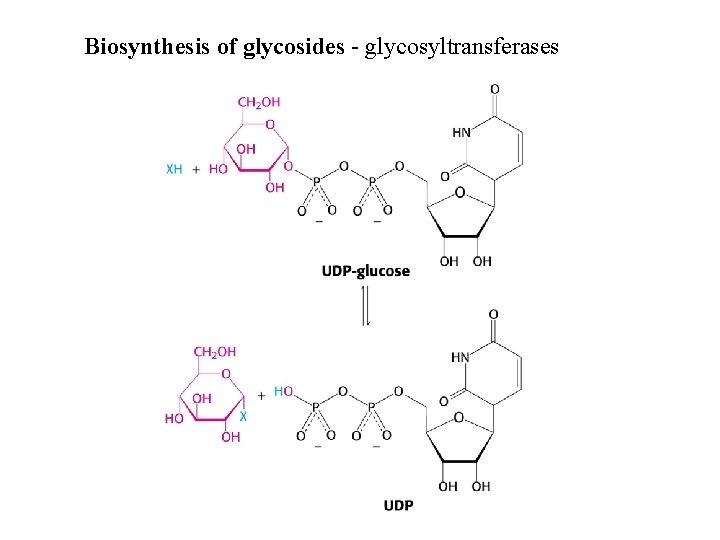
Biosynthesis of glycosides - glycosyltransferases
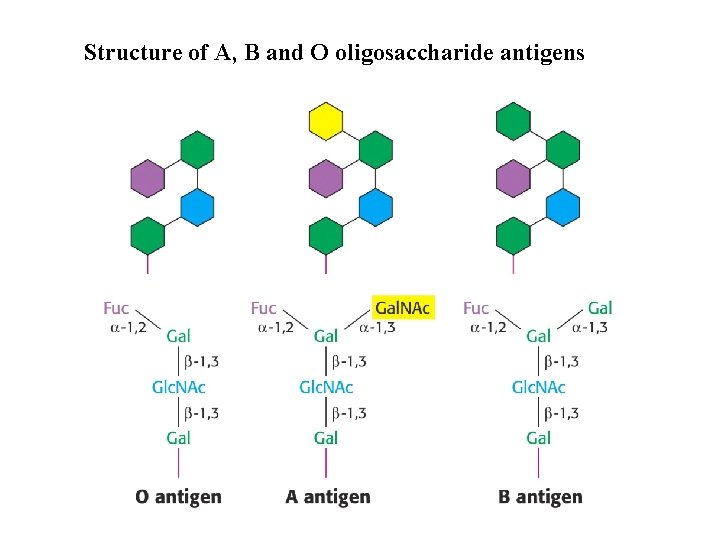
Structure of A, B and O oligosaccharide antigens
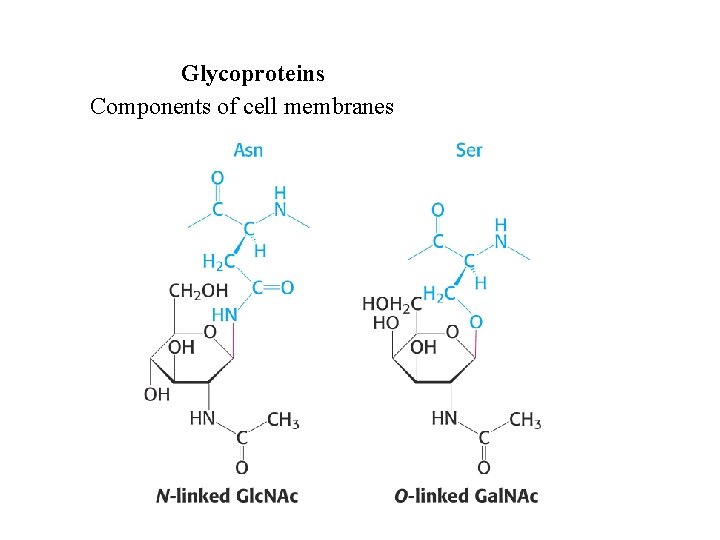
Glycoproteins Components of cell membranes
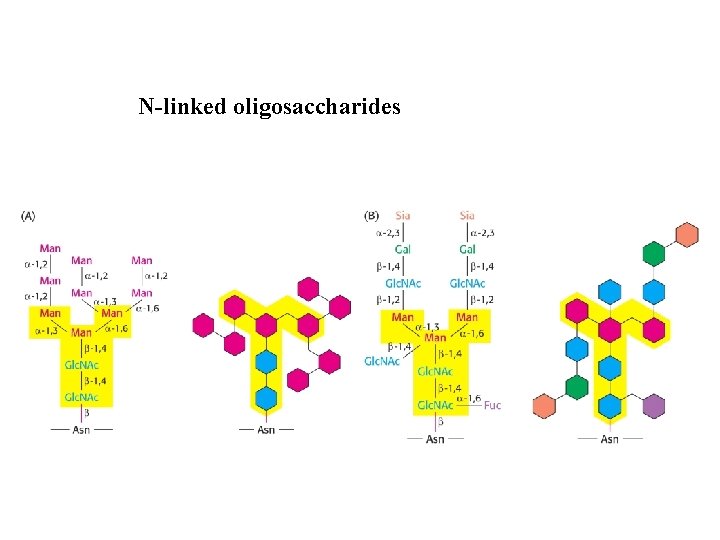
N-linked oligosaccharides
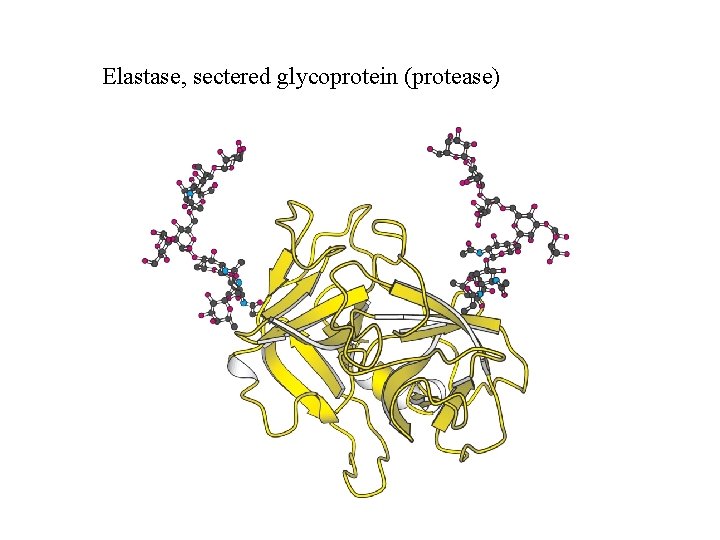
Elastase, sectered glycoprotein (protease)
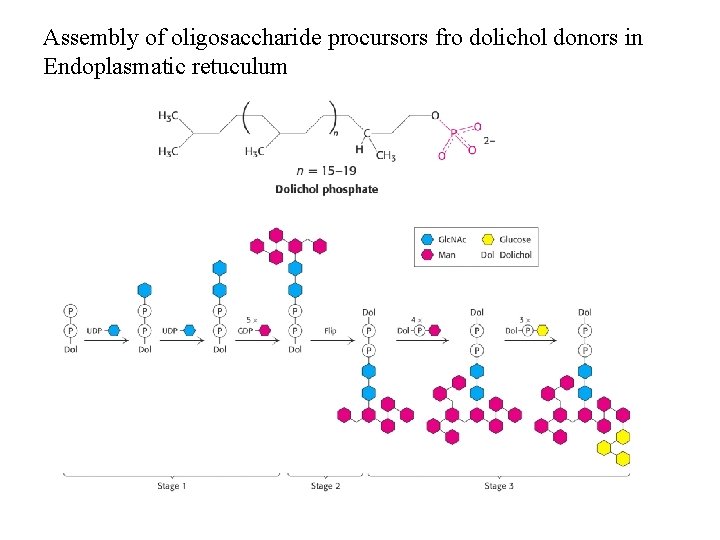
Assembly of oligosaccharide procursors fro dolichol donors in Endoplasmatic retuculum
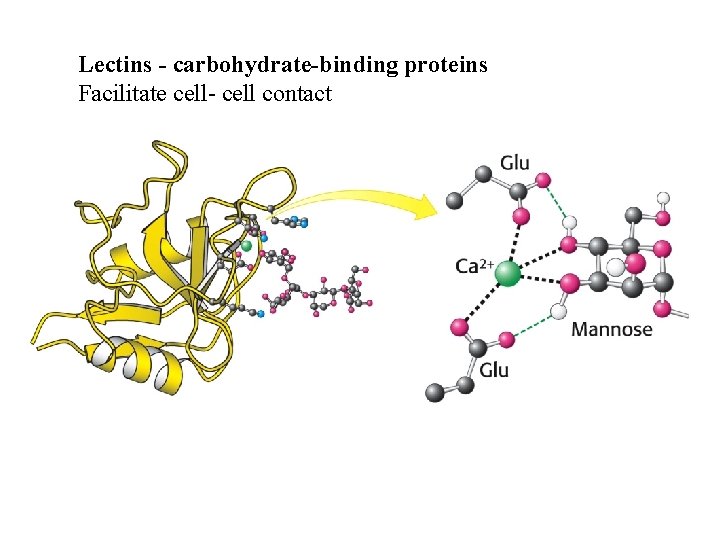
Lectins - carbohydrate-binding proteins Facilitate cell- cell contact
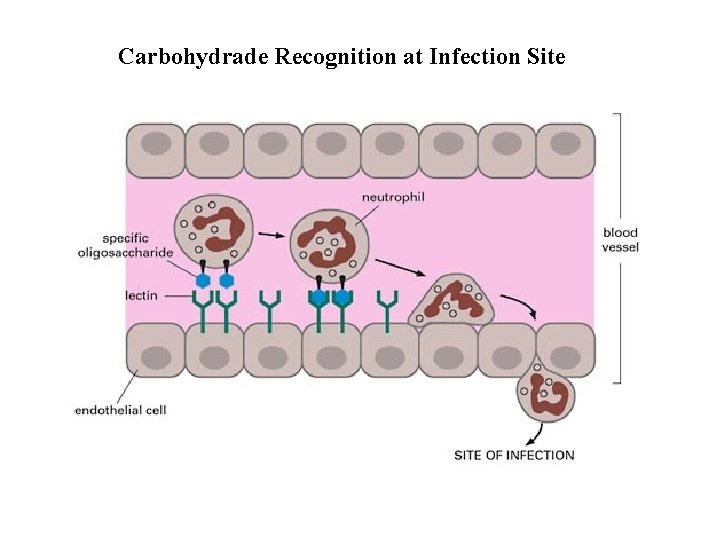
Carbohydrade Recognition at Infection Site
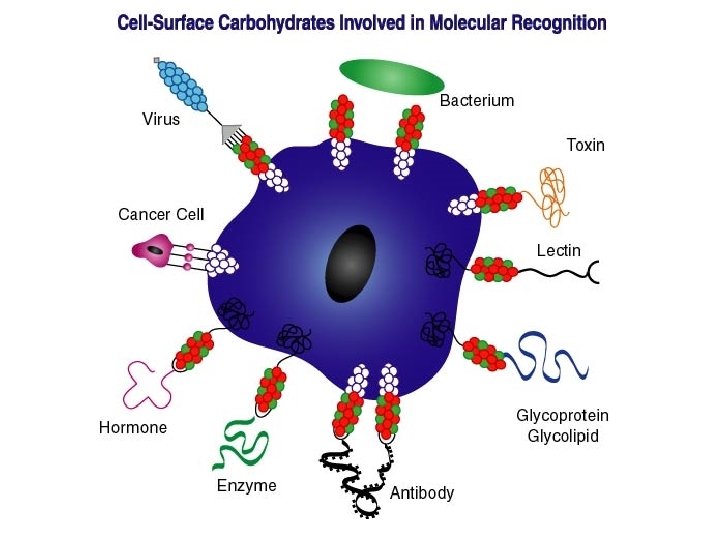
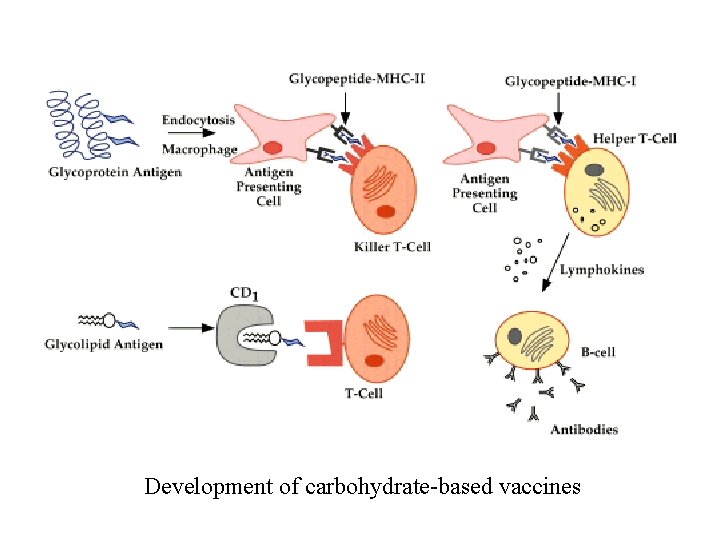
Development of carbohydrate-based vaccines
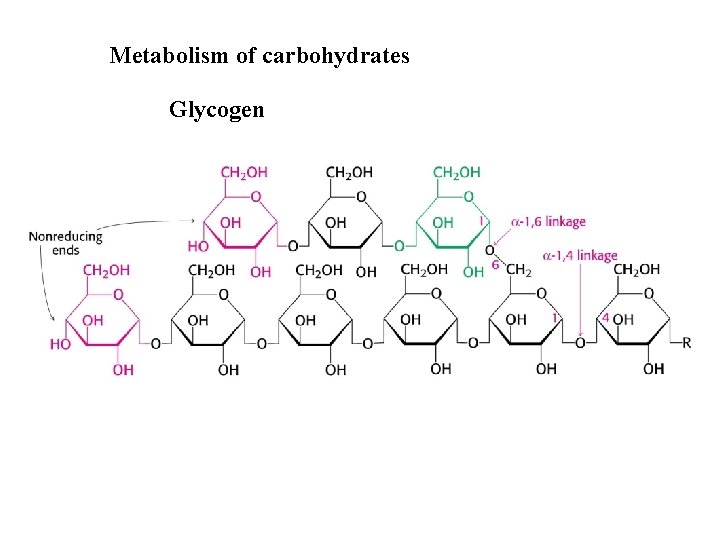
Metabolism of carbohydrates Glycogen
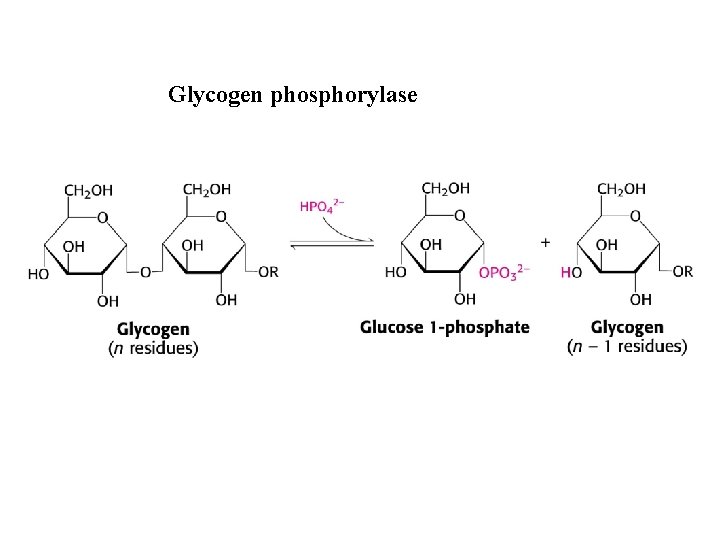
Glycogen phosphorylase
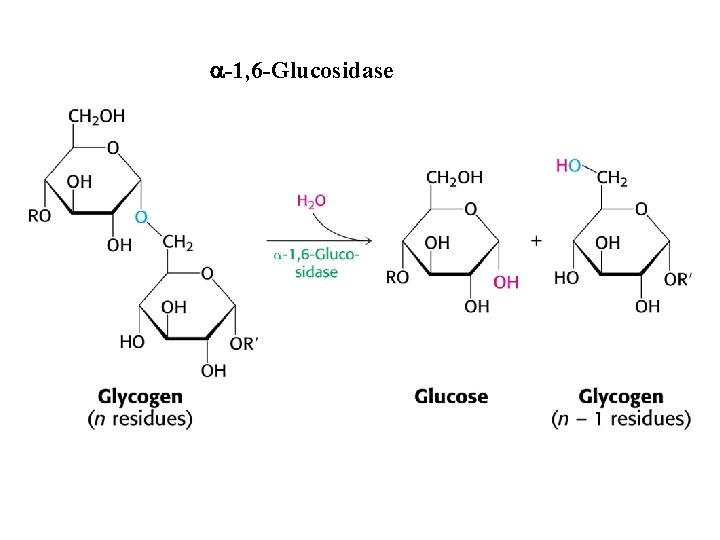
a-1, 6 -Glucosidase
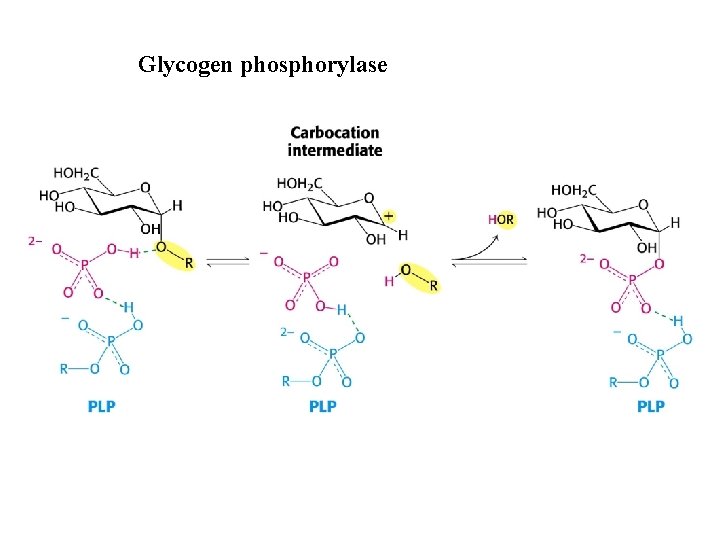
Glycogen phosphorylase
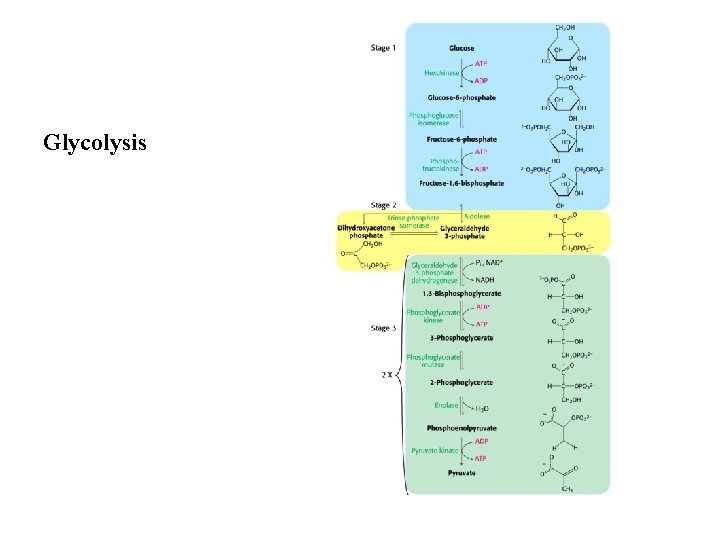
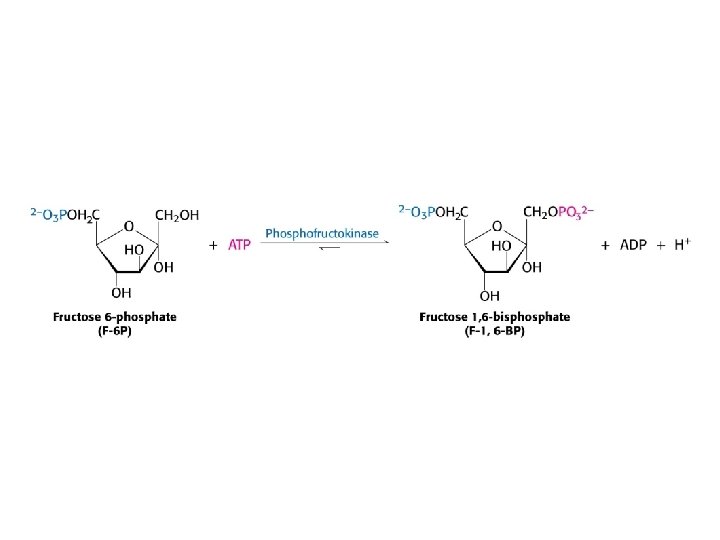
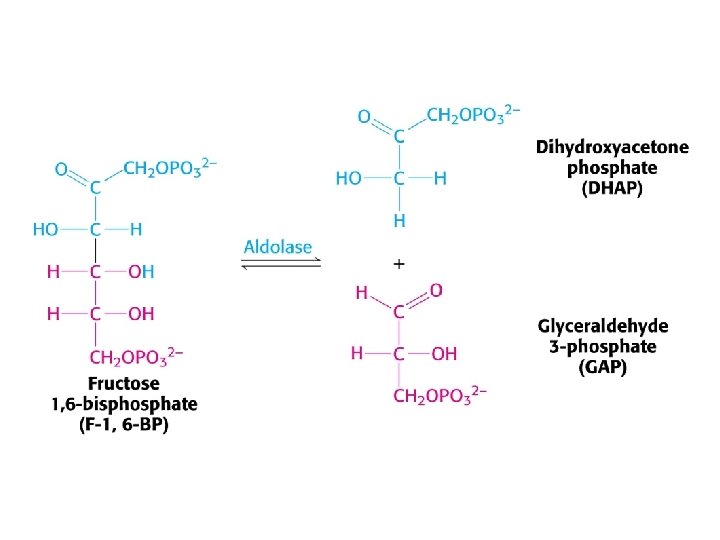
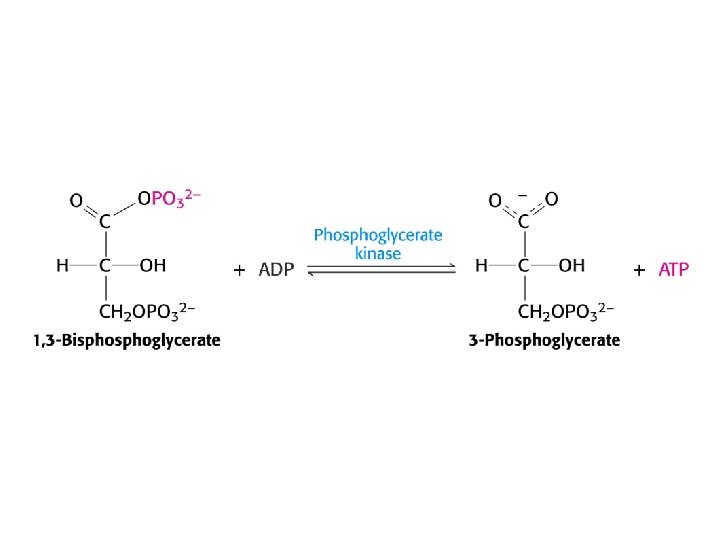
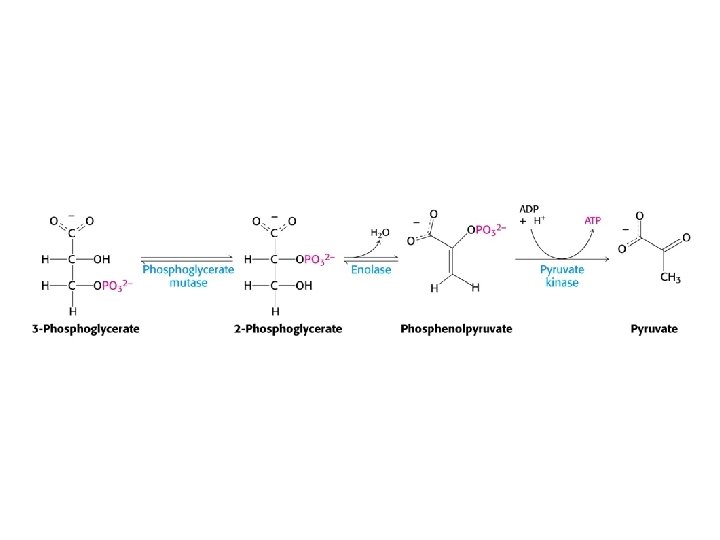
Glycolysis
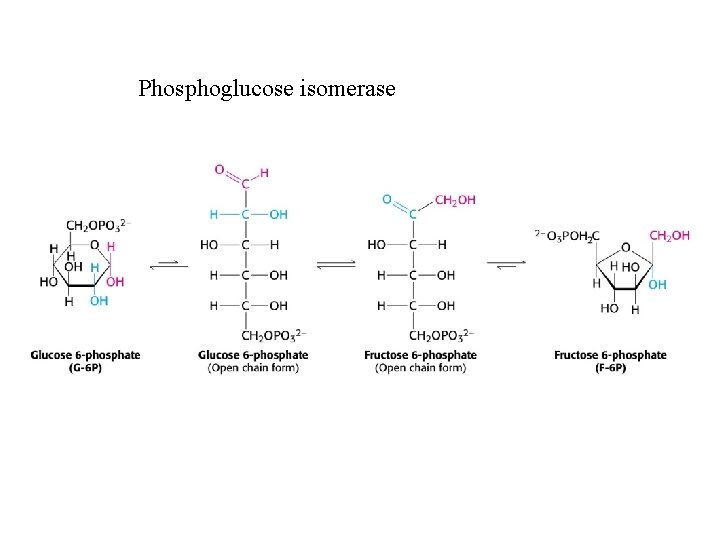
Phosphoglucose isomerase
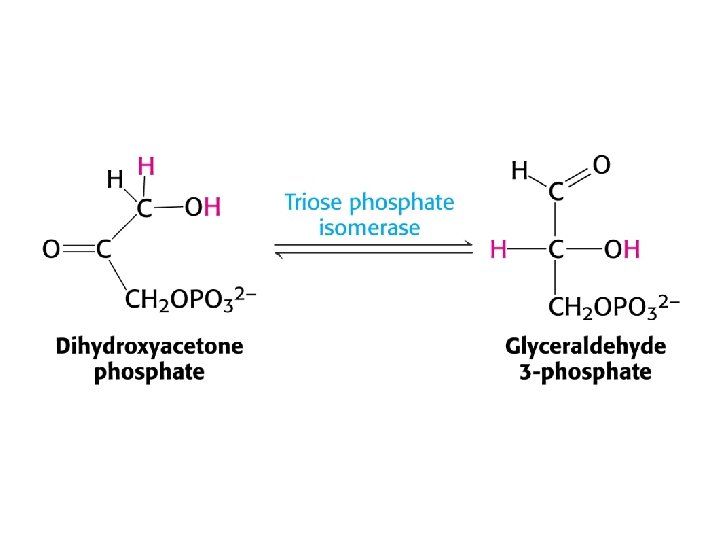
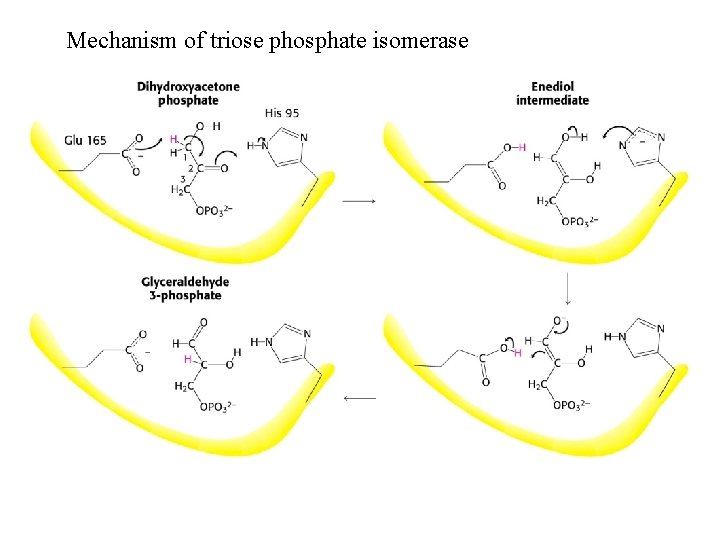
Mechanism of triose phosphate isomerase
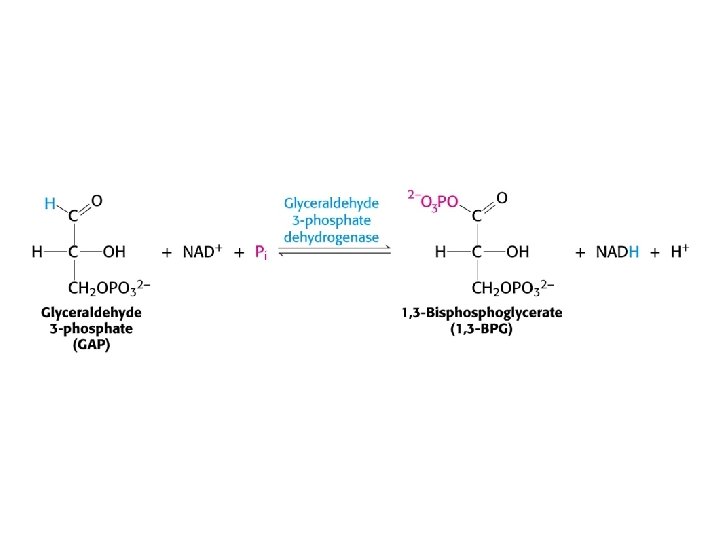
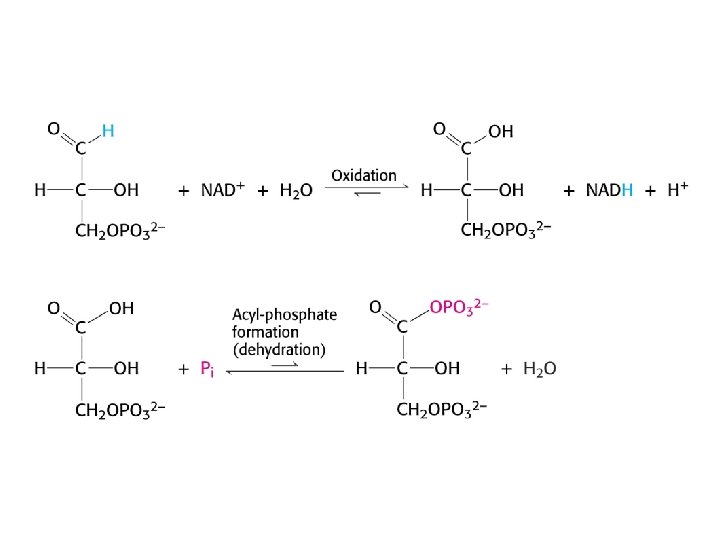
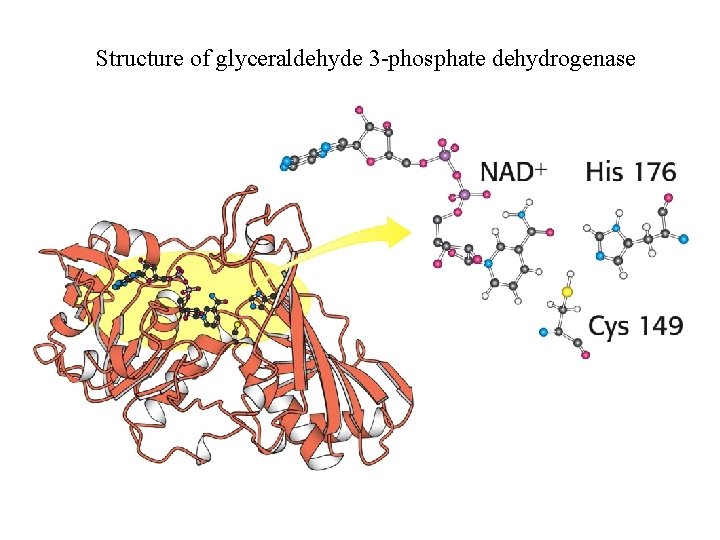
Structure of glyceraldehyde 3 -phosphate dehydrogenase
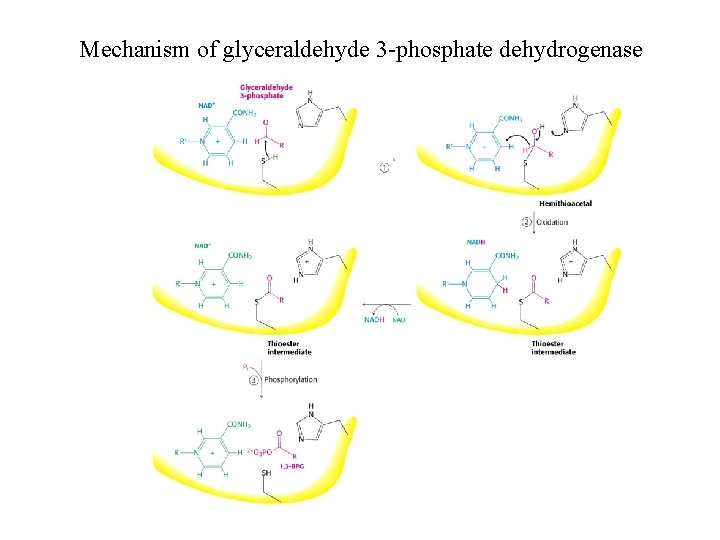
Mechanism of glyceraldehyde 3 -phosphate dehydrogenase
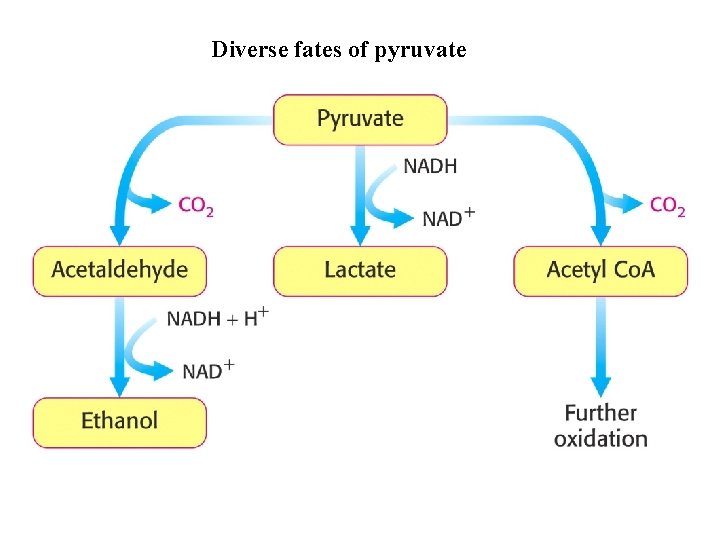
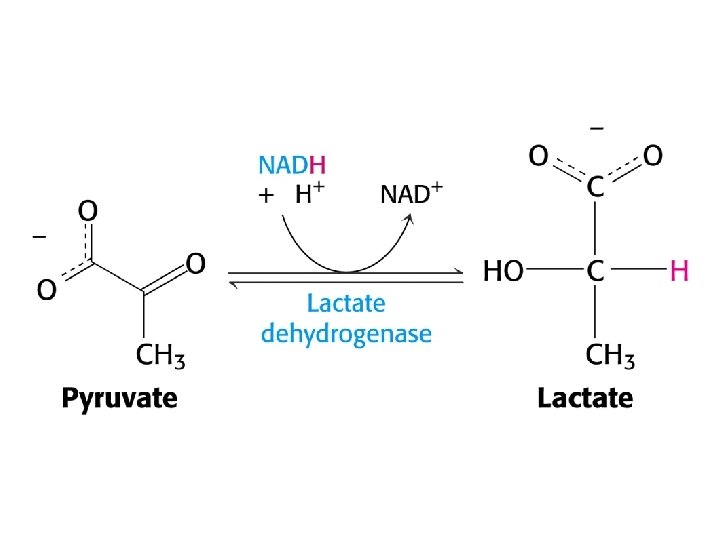
Diverse fates of pyruvate
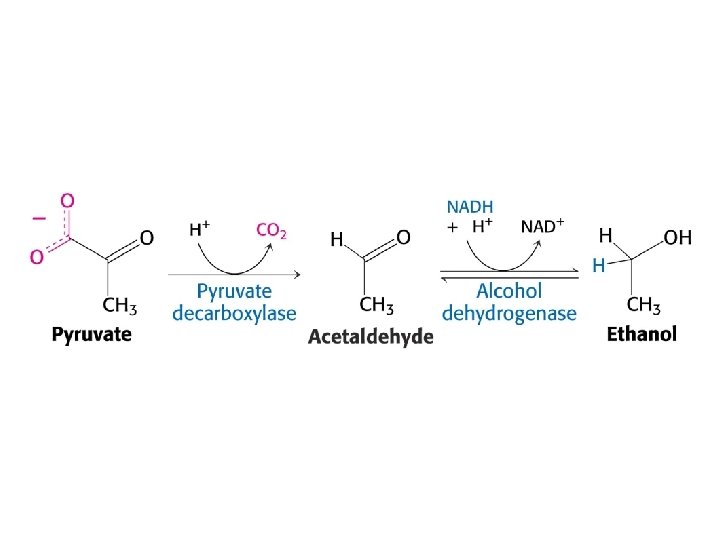
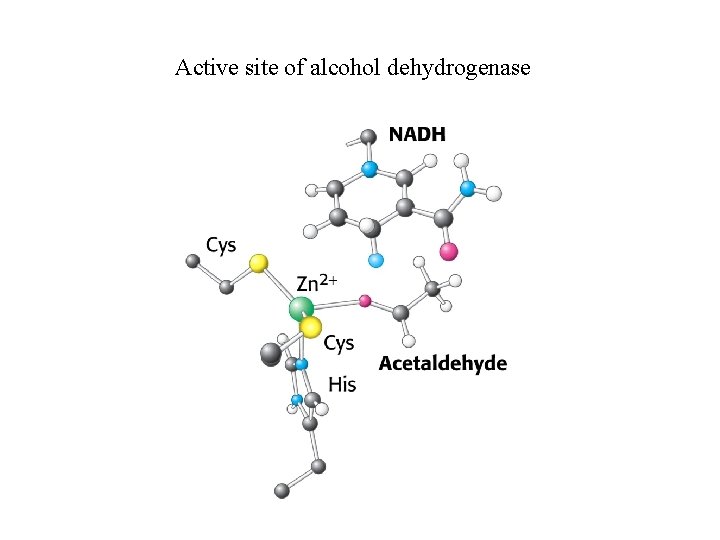
Active site of alcohol dehydrogenase
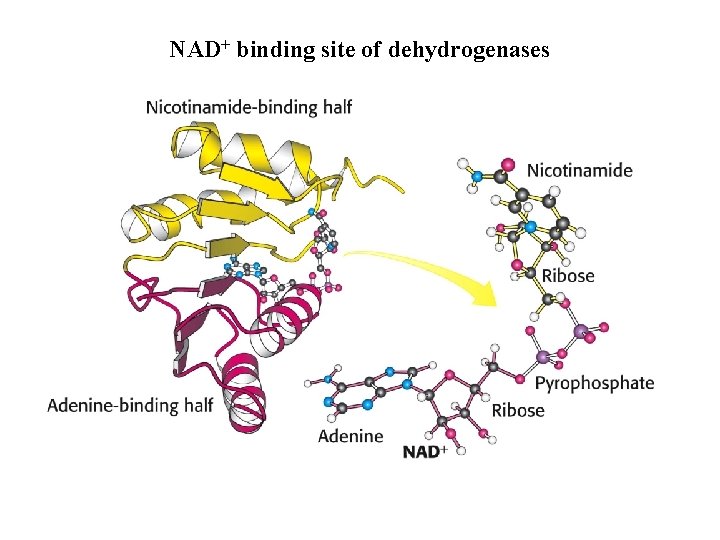
NAD+ binding site of dehydrogenases
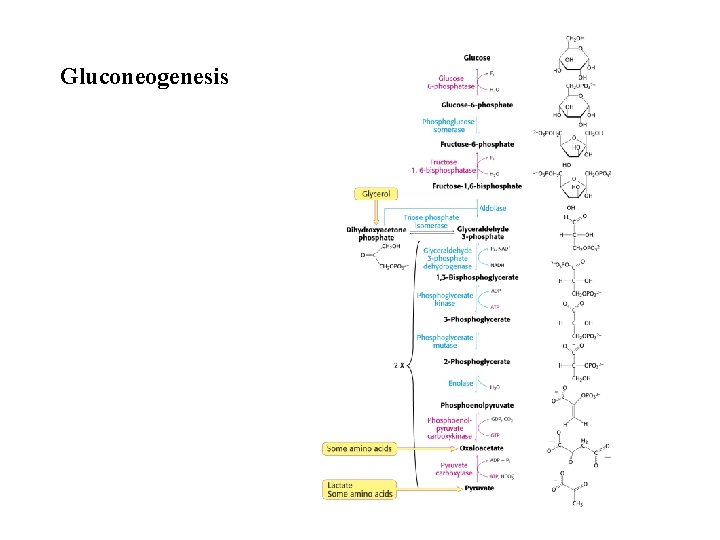
Gluconeogenesis
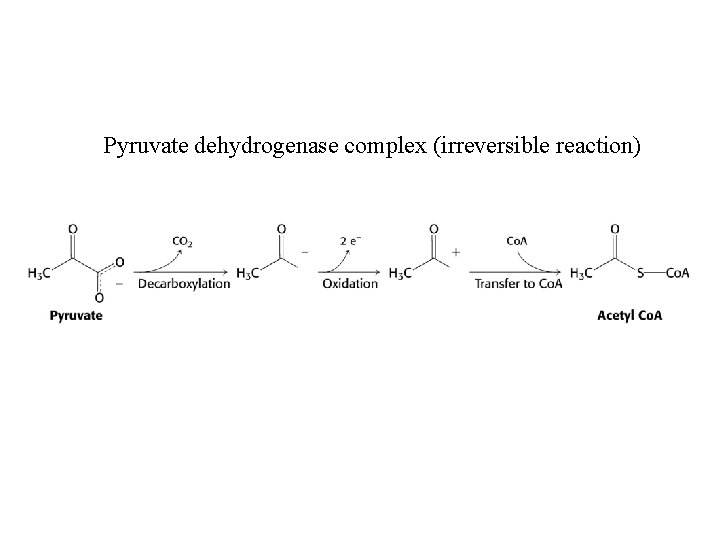
Pyruvate dehydrogenase complex (irreversible reaction)
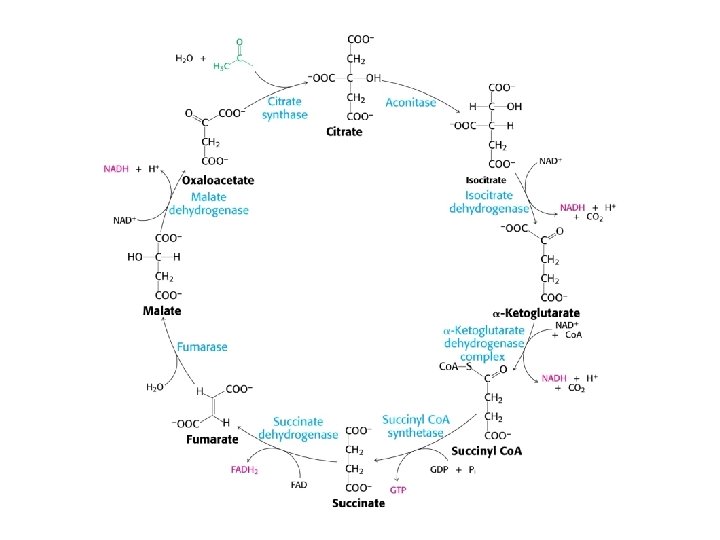
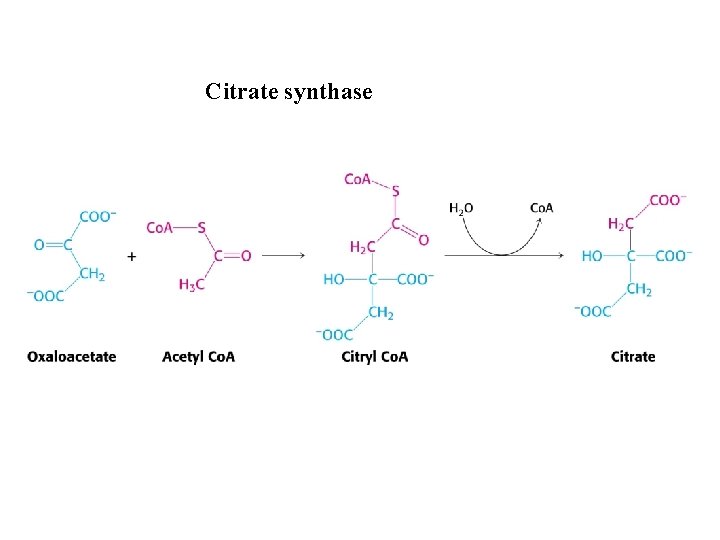
Citrate synthase
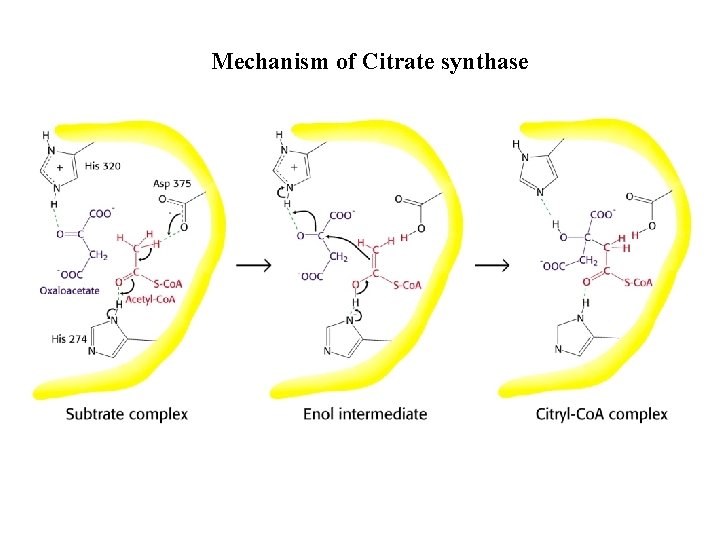
Mechanism of Citrate synthase
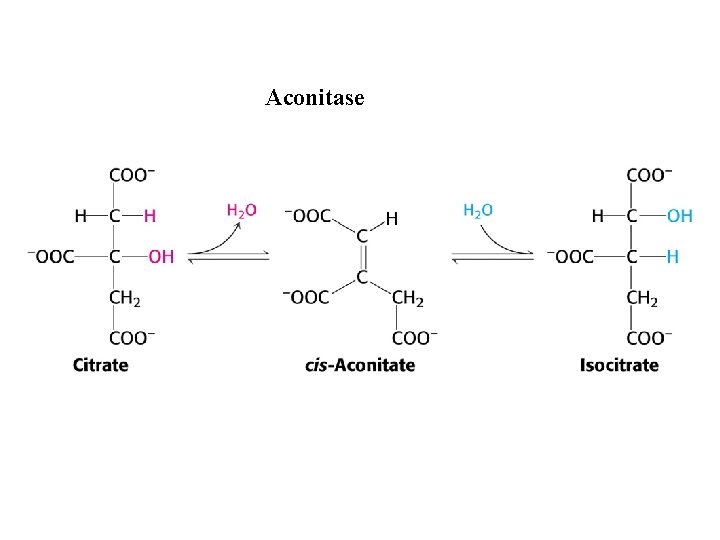
Aconitase
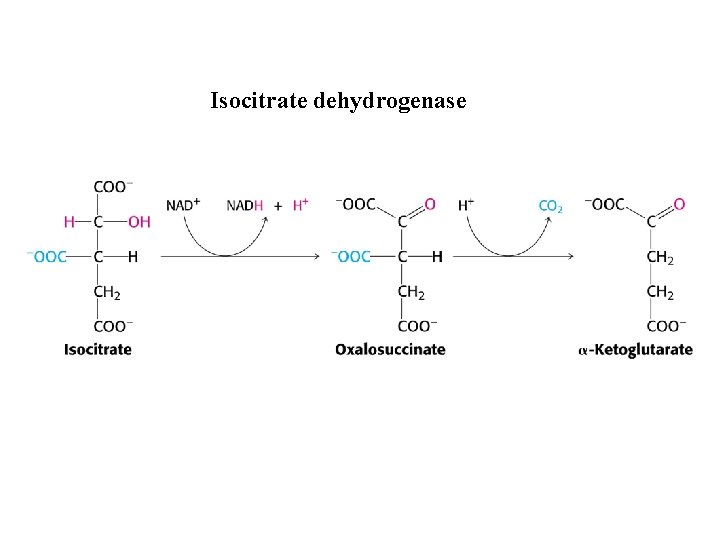
Isocitrate dehydrogenase
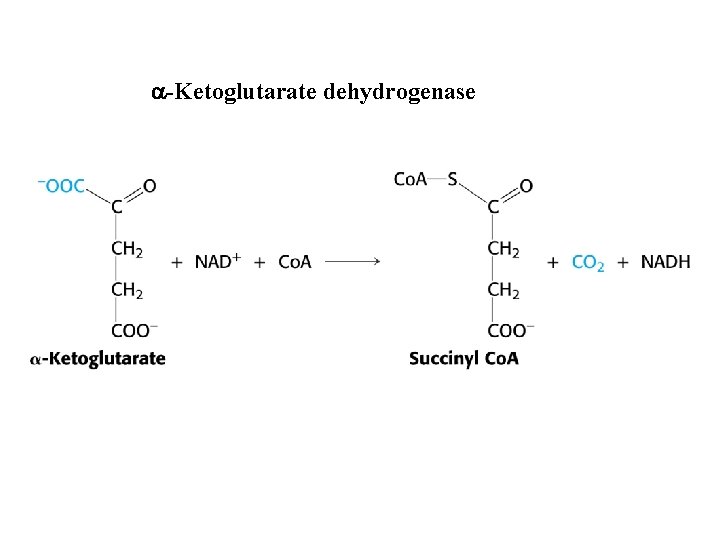
a-Ketoglutarate dehydrogenase
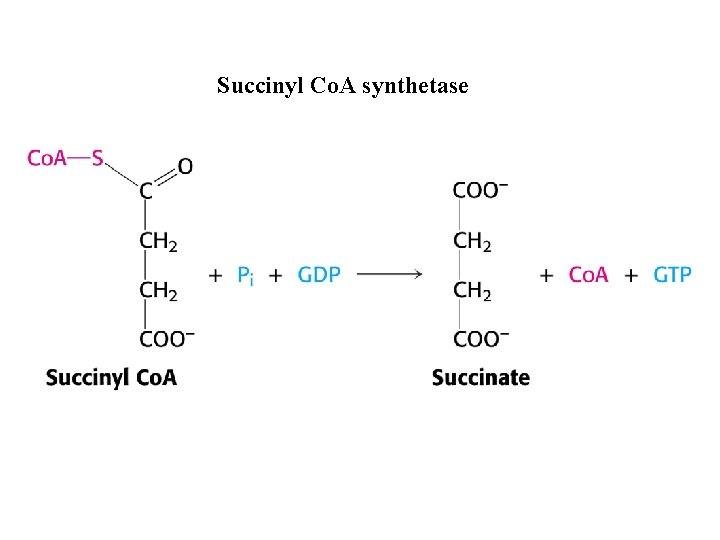
Succinyl Co. A synthetase
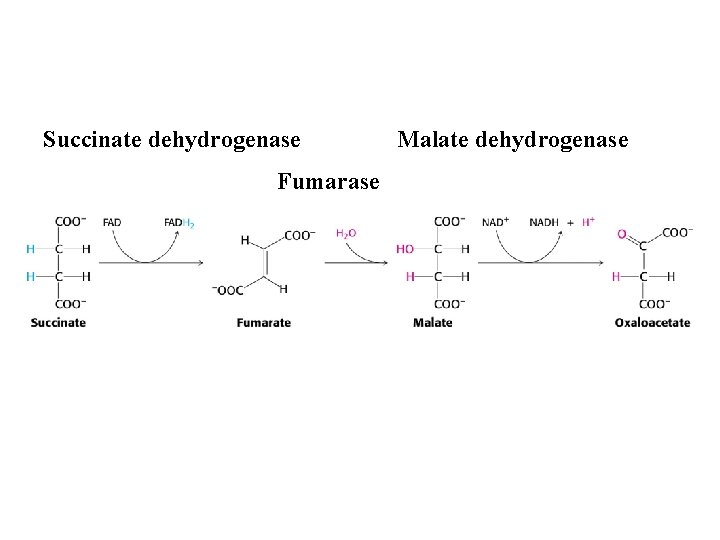
Succinate dehydrogenase Fumarase Malate dehydrogenase

Chemical and enzymatic syntheses of glycosides and oligosaccharides
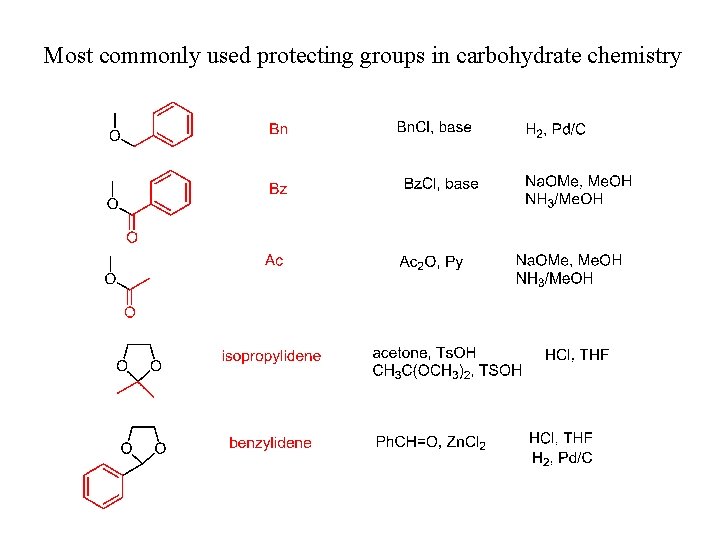
Most commonly used protecting groups in carbohydrate chemistry
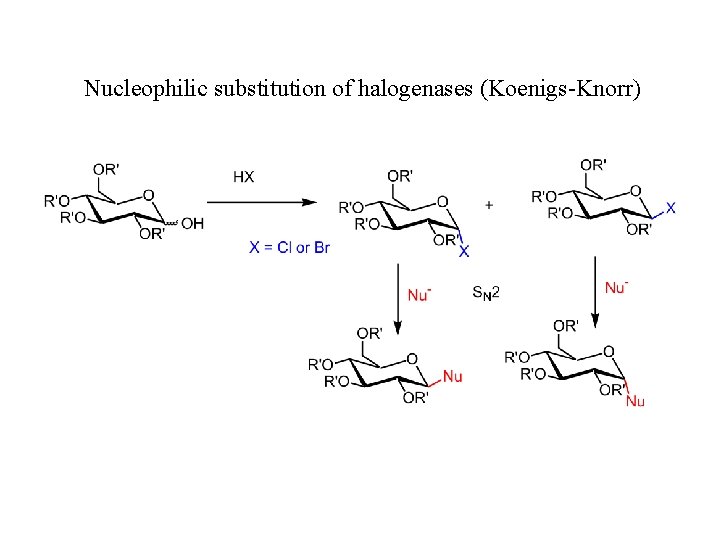
Nucleophilic substitution of halogenases (Koenigs-Knorr)
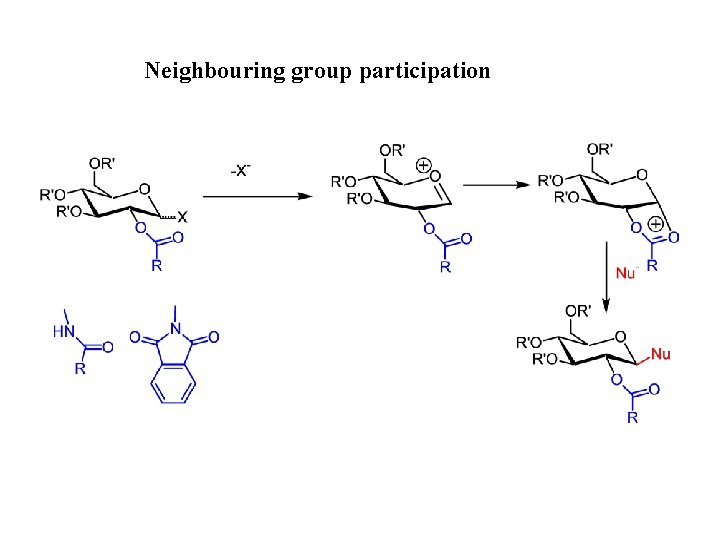
Neighbouring group participation
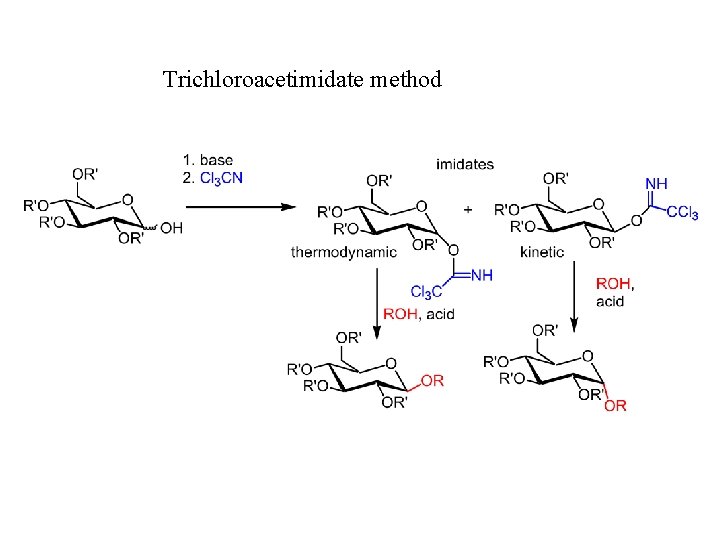
Trichloroacetimidate method
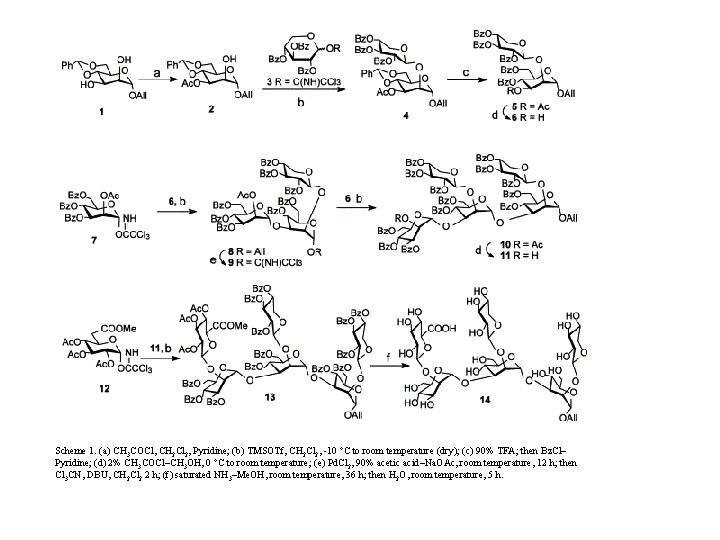
Scheme 1. (a) CH 3 COCl, CH 2 Cl 2, Pyridine; (b) TMSOTf, CH 2 Cl 2, -10 °C to room temperature (dry); (c) 90% TFA; then Bz. Cl– Pyridine; (d) 2% CH 3 COCl–CH 3 OH, 0 °C to room temperature; (e) Pd. Cl 2, 90% acetic acid–Na. OAc, room temperature, 12 h; then Cl 3 CN, DBU, CH 2 Cl 2 2 h; (f) saturated NH 3–Me. OH, room temperature, 36 h; then H 2 O, room temperature, 5 h.
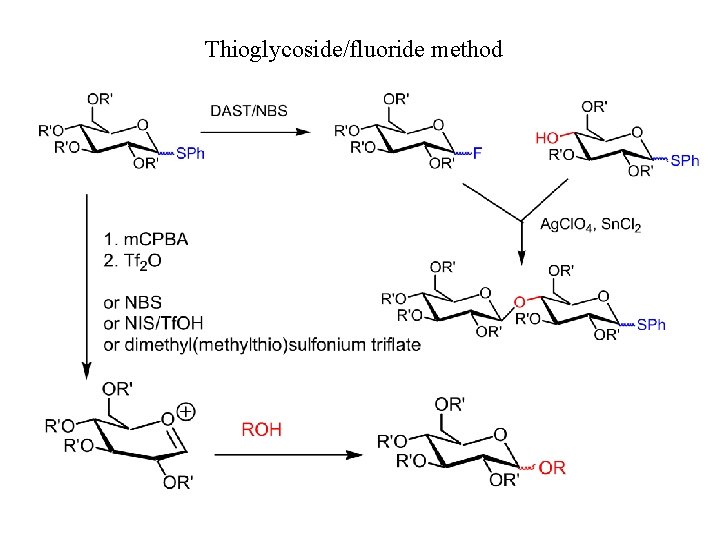
Thioglycoside/fluoride method
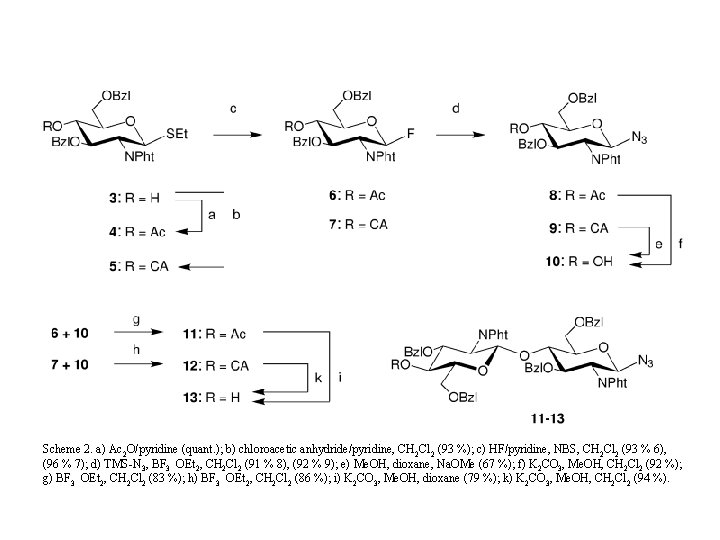
Scheme 2. a) Ac 2 O/pyridine (quant. ); b) chloroacetic anhydride/pyridine, CH 2 Cl 2 (93 %); c) HF/pyridine, NBS, CH 2 Cl 2 (93 % 6), (96 % 7); d) TMS-N 3, BF 3 OEt 2, CH 2 Cl 2 (91 % 8), (92 % 9); e) Me. OH, dioxane, Na. OMe (67 %); f) K 2 CO 3, Me. OH, CH 2 Cl 2 (92 %); g) BF 3 OEt 2, CH 2 Cl 2 (83 %); h) BF 3 OEt 2, CH 2 Cl 2 (86 %); i) K 2 CO 3, Me. OH, dioxane (79 %); k) K 2 CO 3, Me. OH, CH 2 Cl 2 (94 %).
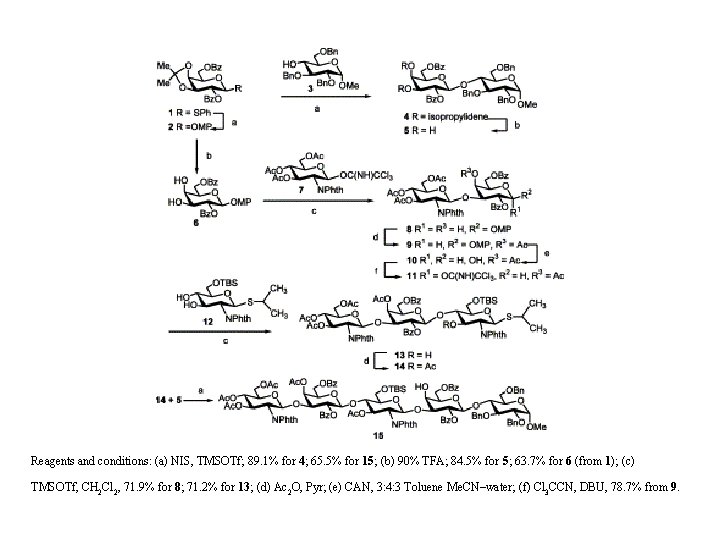
Reagents and conditions: (a) NIS, TMSOTf; 89. 1% for 4; 65. 5% for 15; (b) 90% TFA; 84. 5% for 5; 63. 7% for 6 (from 1); (c) TMSOTf, CH 2 Cl 2, 71. 9% for 8; 71. 2% for 13; (d) Ac 2 O, Pyr; (e) CAN, 3: 4: 3 Toluene Me. CN–water; (f) Cl 3 CCN, DBU, 78. 7% from 9.
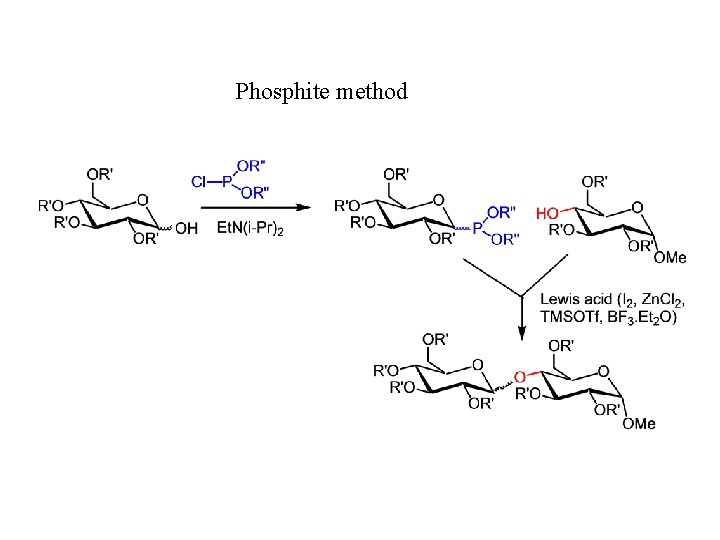
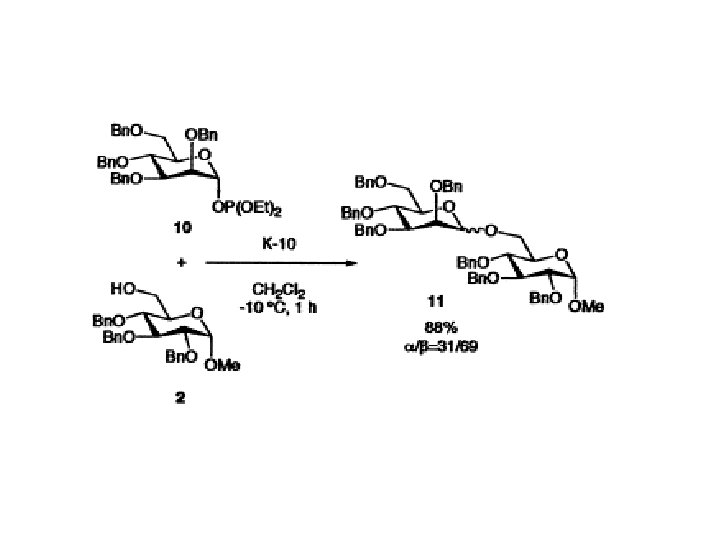
Phosphite method
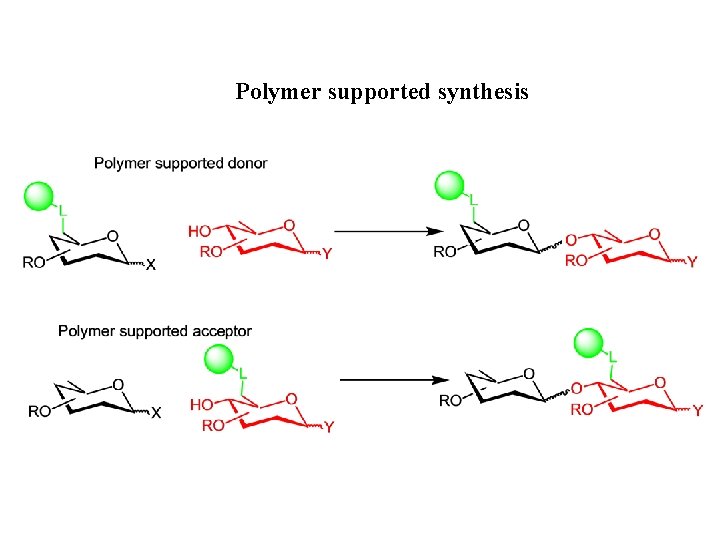
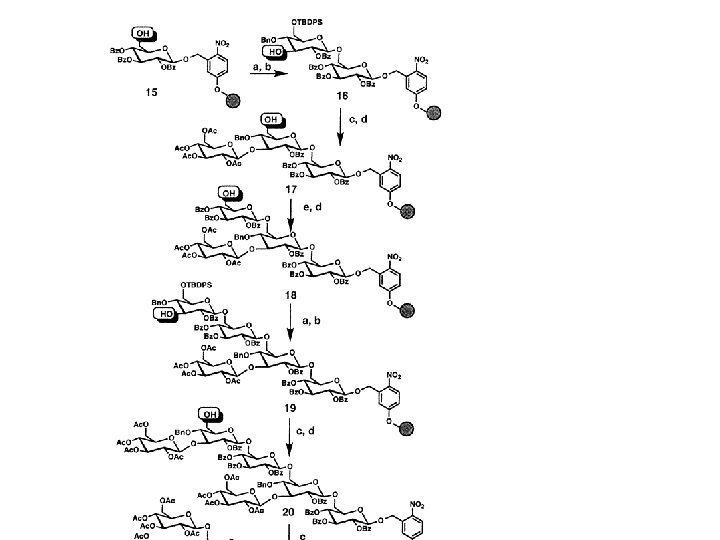
Polymer supported synthesis
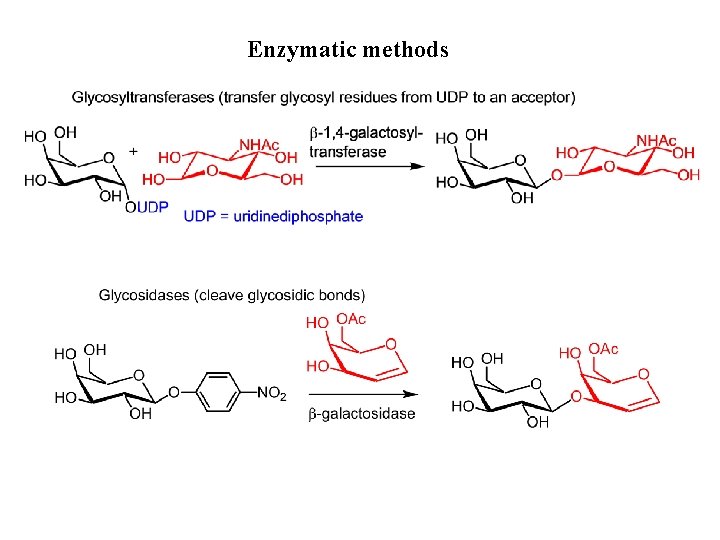
Enzymatic methods
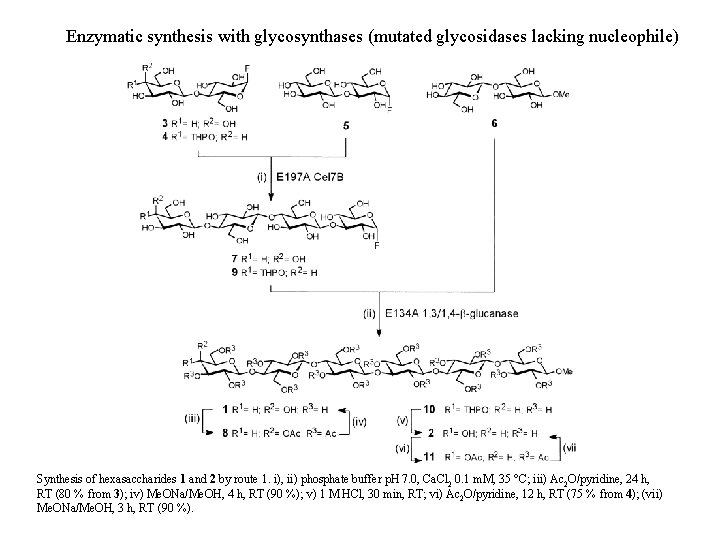
Enzymatic synthesis with glycosynthases (mutated glycosidases lacking nucleophile) Synthesis of hexasaccharides 1 and 2 by route 1. i), ii) phosphate buffer p. H 7. 0, Ca. Cl 2 0. 1 m. M, 35 °C; iii) Ac 2 O/pyridine, 24 h, RT (80 % from 3); iv) Me. ONa/Me. OH, 4 h, RT (90 %); v) 1 M HCl, 30 min, RT; vi) Ac 2 O/pyridine, 12 h, RT (75 % from 4); (vii) Me. ONa/Me. OH, 3 h, RT (90 %).
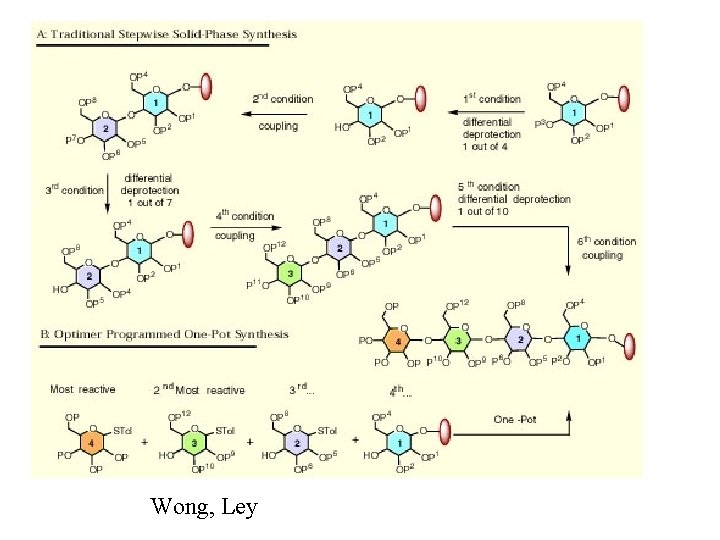
Wong, Ley
- Slides: 79