Carbohydrates Lecture 3 What is a Carbohydrate Aldehyde

Carbohydrates Lecture 3
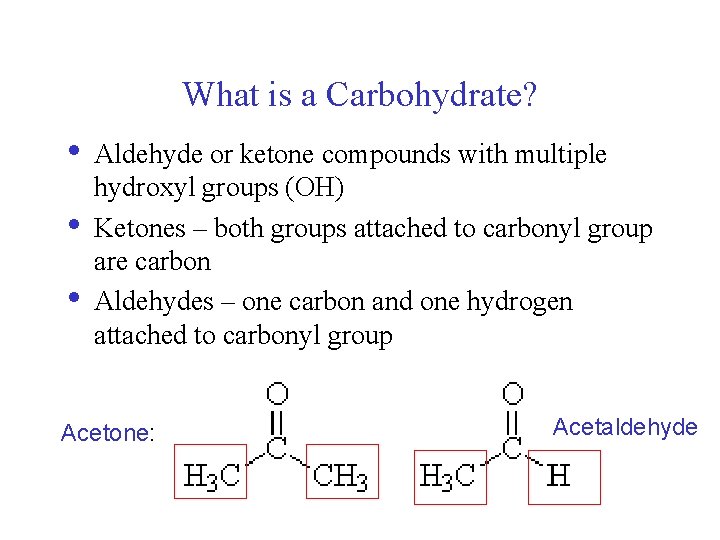
What is a Carbohydrate? • • • Aldehyde or ketone compounds with multiple hydroxyl groups (OH) Ketones – both groups attached to carbonyl group are carbon Aldehydes – one carbon and one hydrogen attached to carbonyl group Acetone: Acetaldehyde
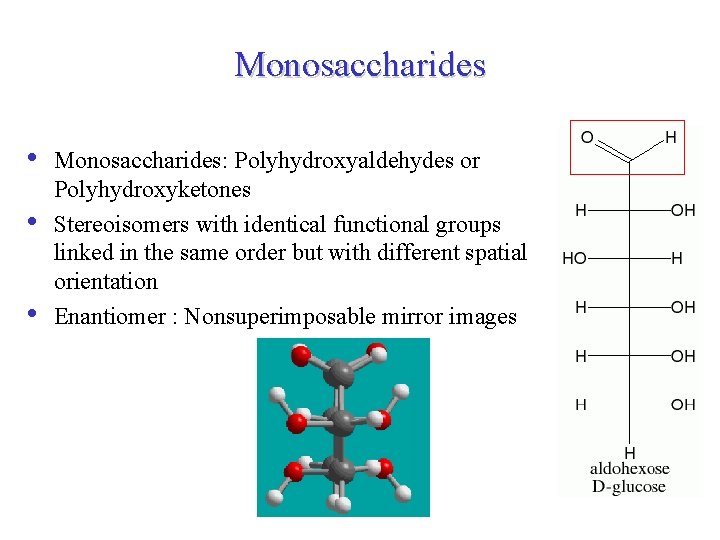
Monosaccharides • • • Monosaccharides: Polyhydroxyaldehydes or Polyhydroxyketones Stereoisomers with identical functional groups linked in the same order but with different spatial orientation Enantiomer : Nonsuperimposable mirror images
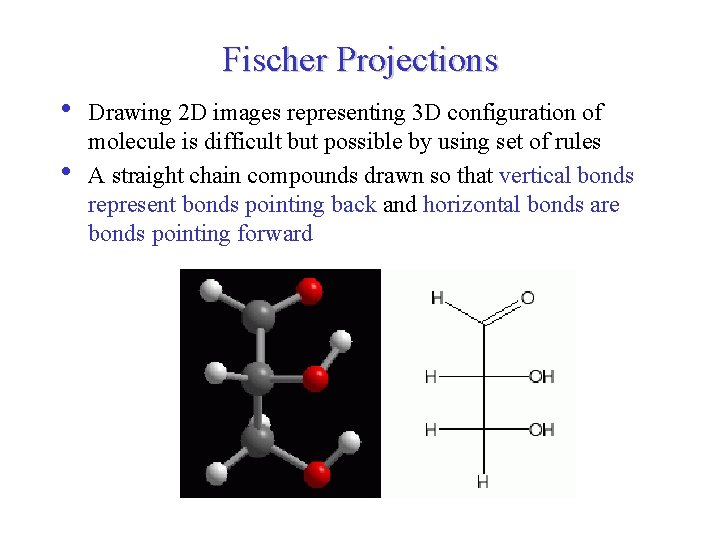
Fischer Projections • • Drawing 2 D images representing 3 D configuration of molecule is difficult but possible by using set of rules A straight chain compounds drawn so that vertical bonds represent bonds pointing back and horizontal bonds are bonds pointing forward
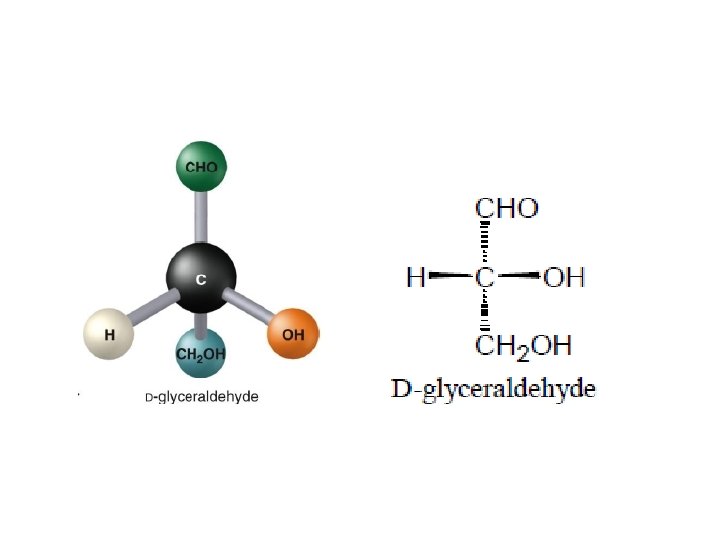
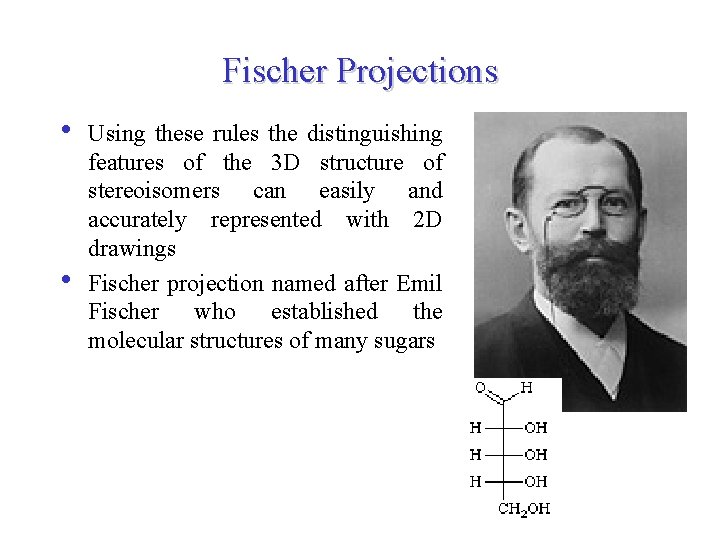
Fischer Projections • • Using these rules the distinguishing features of the 3 D structure of stereoisomers can easily and accurately represented with 2 D drawings Fischer projection named after Emil Fischer who established the molecular structures of many sugars
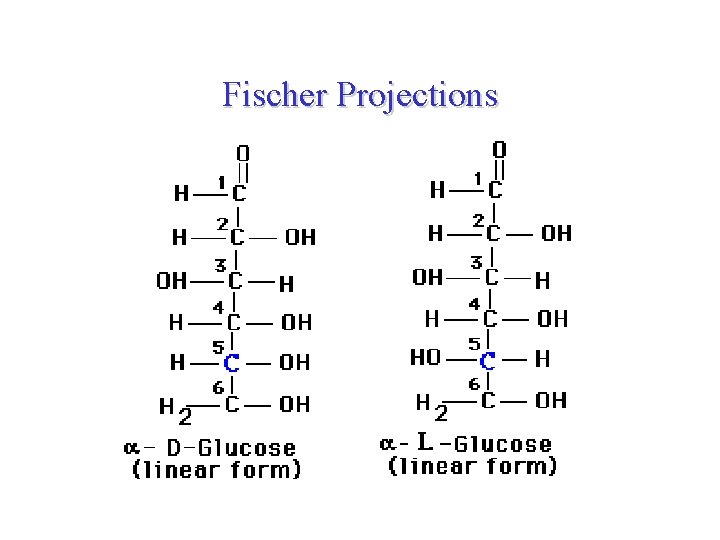
Fischer Projections galactose Glucose
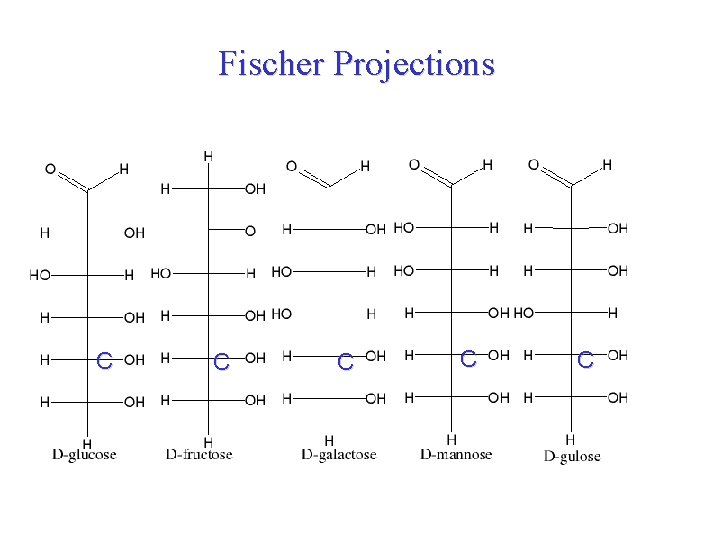
Fischer Projections galactose C C C Galactose C C Glucose
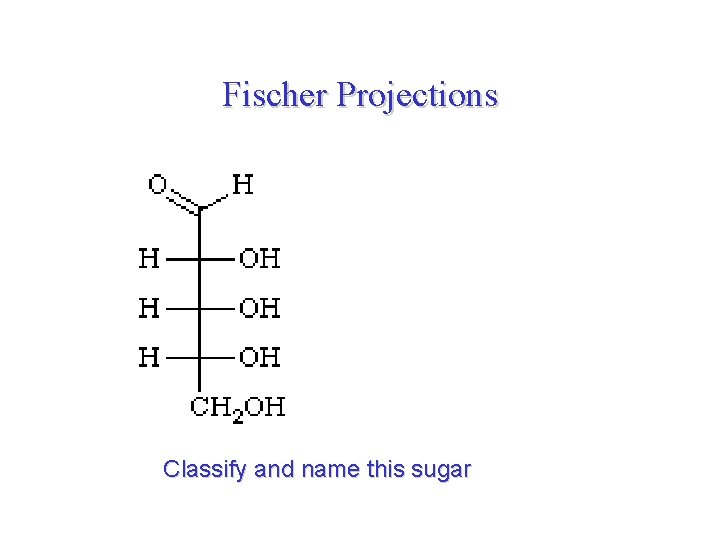
Fischer Projections Classify and name this sugar
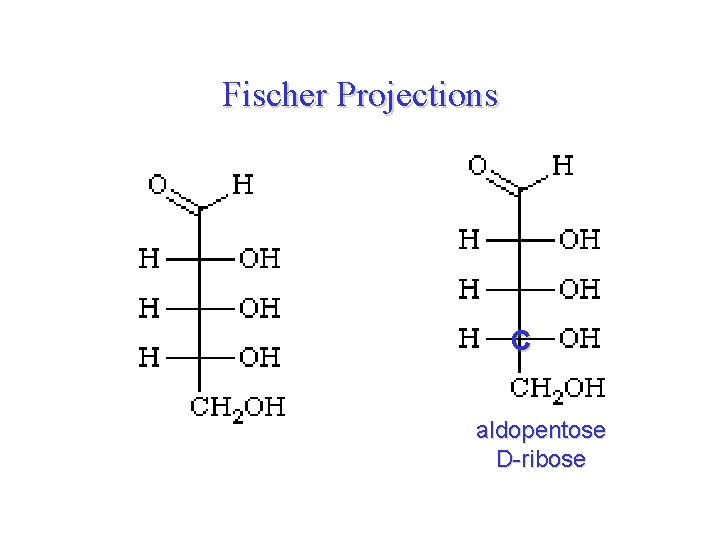
Fischer Projections C aldopentose D-ribose
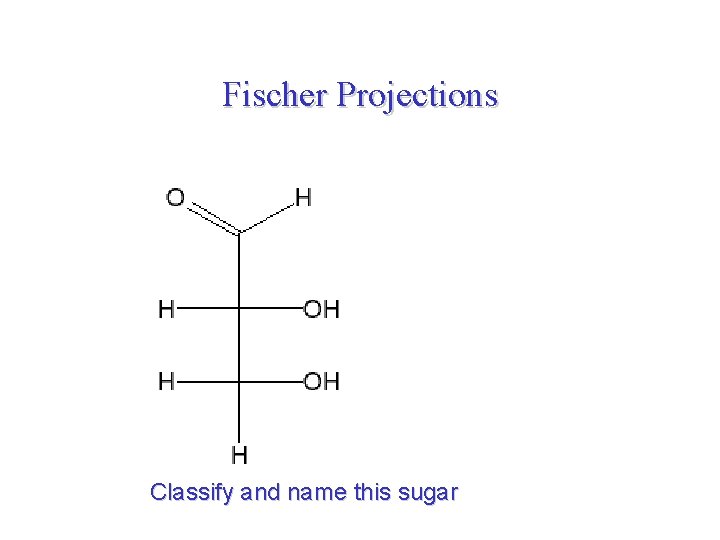
Fischer Projections Classify and name this sugar
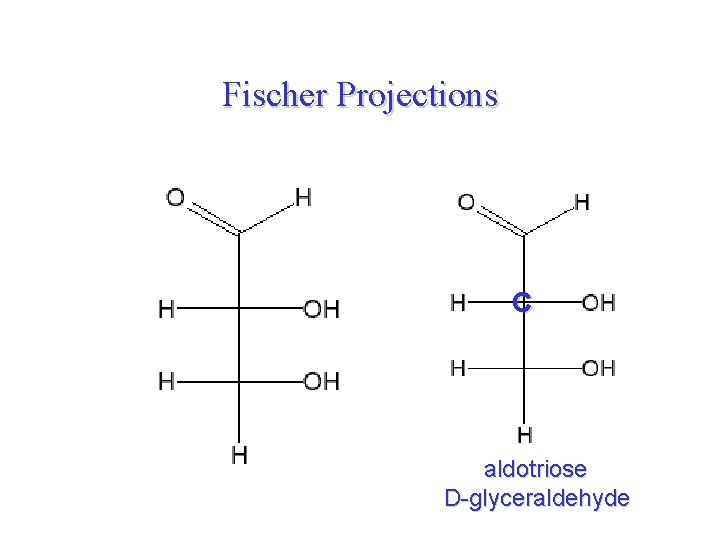
Fischer Projections C aldotriose D-glyceraldehyde
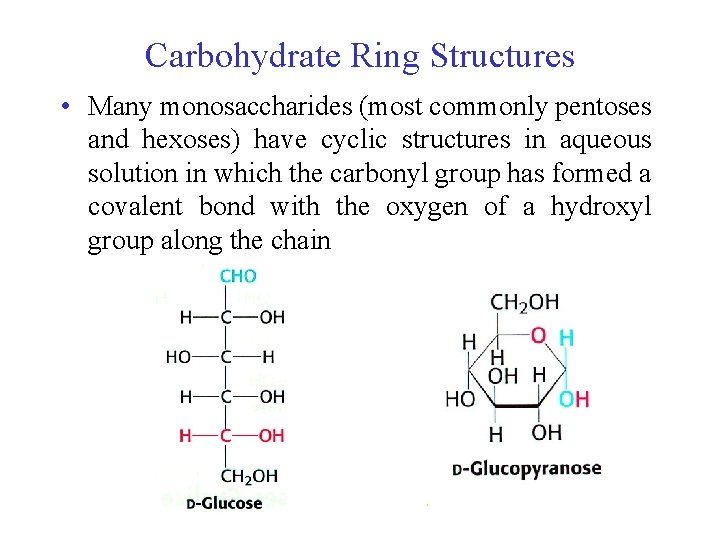
Carbohydrate Ring Structures • Many monosaccharides (most commonly pentoses and hexoses) have cyclic structures in aqueous solution in which the carbonyl group has formed a covalent bond with the oxygen of a hydroxyl group along the chain
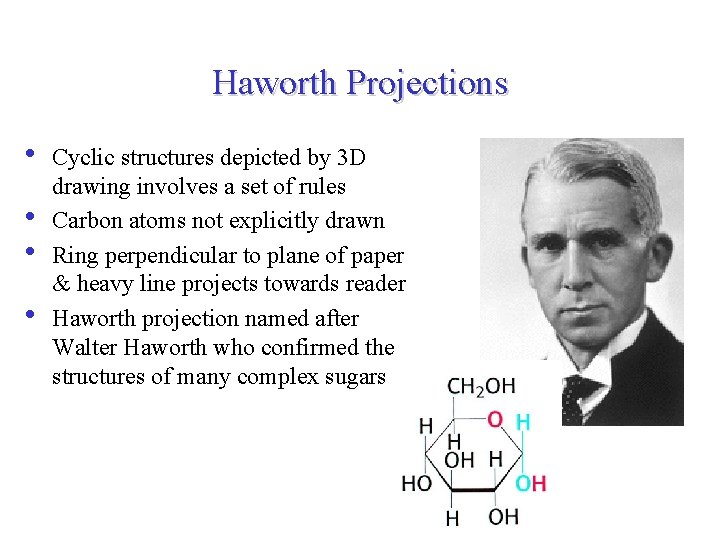
Haworth Projections • • Cyclic structures depicted by 3 D drawing involves a set of rules Carbon atoms not explicitly drawn Ring perpendicular to plane of paper & heavy line projects towards reader Haworth projection named after Walter Haworth who confirmed the structures of many complex sugars
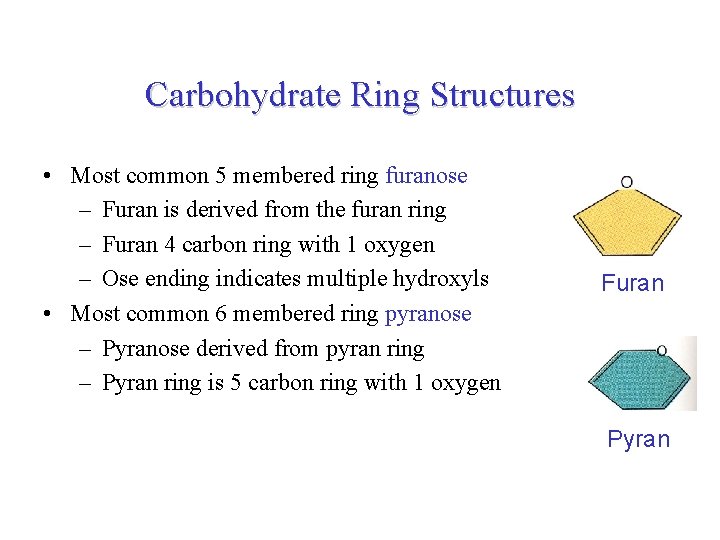
Carbohydrate Ring Structures • Most common 5 membered ring furanose – Furan is derived from the furan ring – Furan 4 carbon ring with 1 oxygen – Ose ending indicates multiple hydroxyls • Most common 6 membered ring pyranose – Pyranose derived from pyran ring – Pyran ring is 5 carbon ring with 1 oxygen Furan Pyran
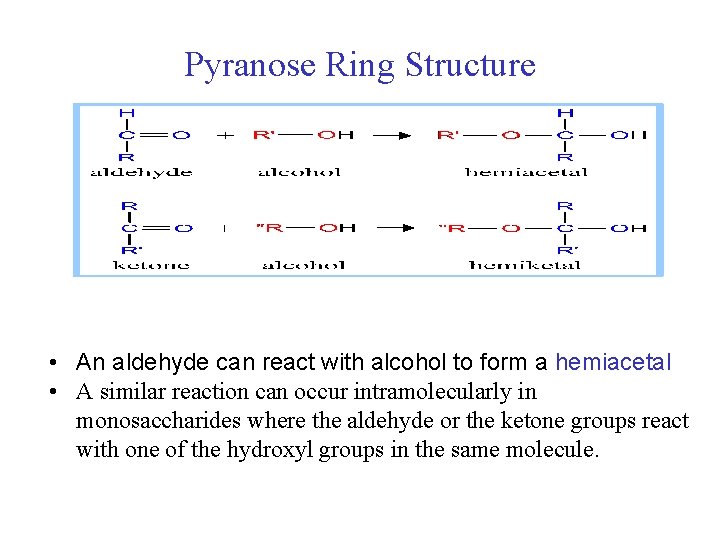
Pyranose Ring Structure • An aldehyde can react with alcohol to form a hemiacetal • A similar reaction can occur intramolecularly in monosaccharides where the aldehyde or the ketone groups react with one of the hydroxyl groups in the same molecule.
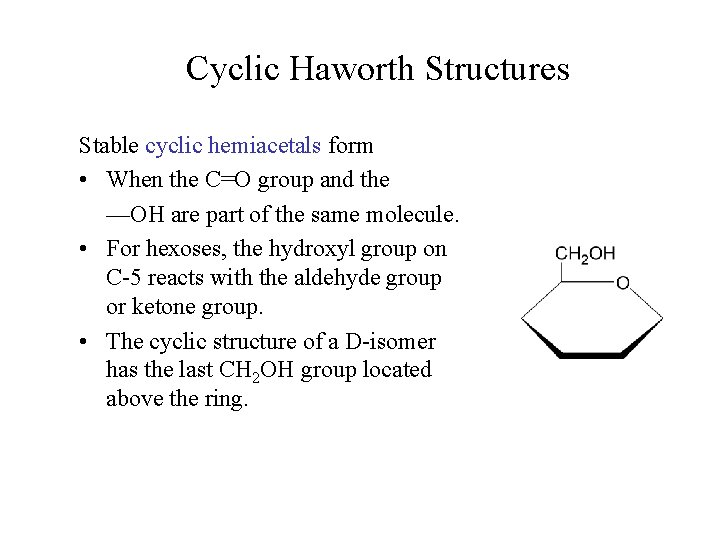
Cyclic Haworth Structures Stable cyclic hemiacetals form • When the C=O group and the —OH are part of the same molecule. • For hexoses, the hydroxyl group on C-5 reacts with the aldehyde group or ketone group. • The cyclic structure of a D-isomer has the last CH 2 OH group located above the ring.
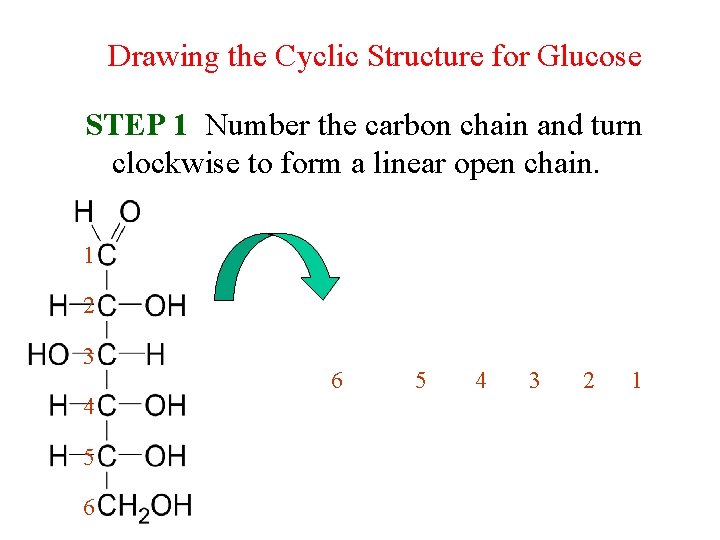
Drawing the Cyclic Structure for Glucose STEP 1 Number the carbon chain and turn clockwise to form a linear open chain. 1 2 3 4 5 6 6 5 4 3 2 1
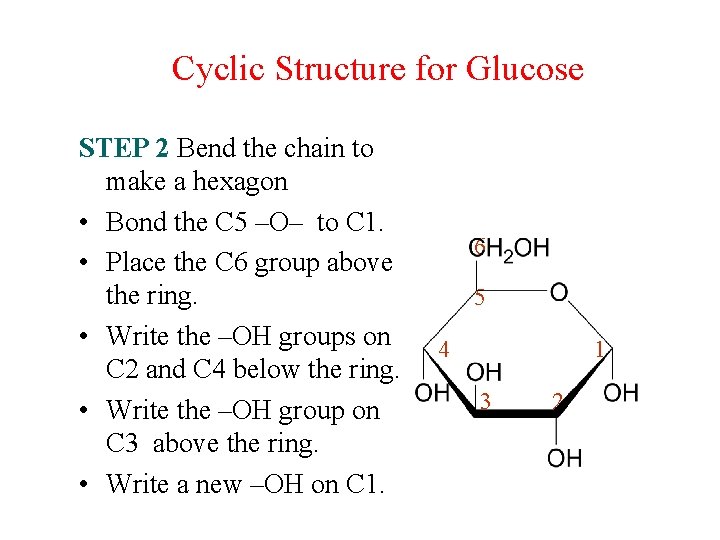
Cyclic Structure for Glucose STEP 2 Bend the chain to make a hexagon • Bond the C 5 –O– to C 1. • Place the C 6 group above the ring. • Write the –OH groups on C 2 and C 4 below the ring. • Write the –OH group on C 3 above the ring. • Write a new –OH on C 1. 6 5 4 1 3 2
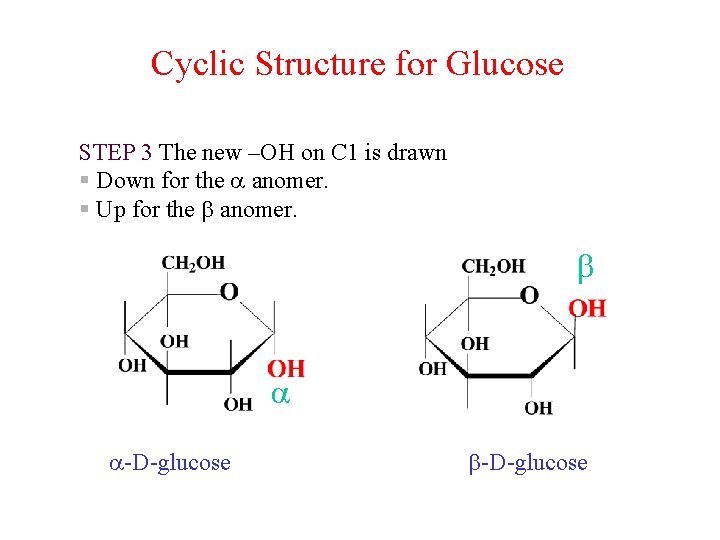
Cyclic Structure for Glucose STEP 3 The new –OH on C 1 is drawn § Down for the anomer. § Up for the anomer. -D-glucose
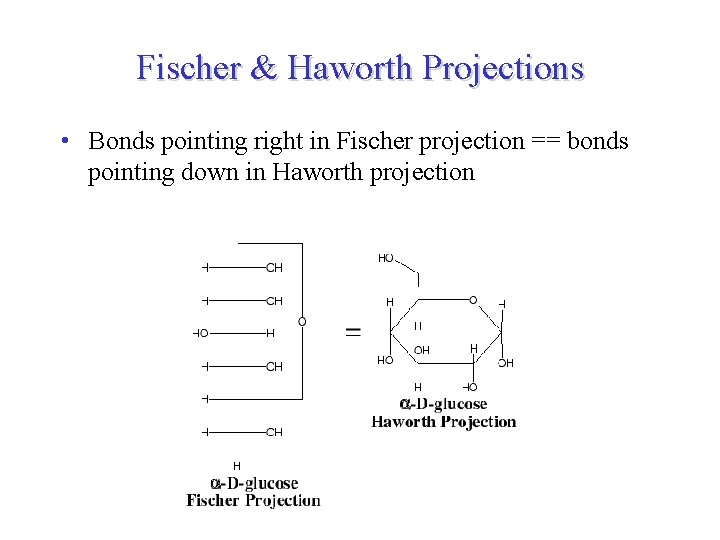
Fischer & Haworth Projections • Bonds pointing right in Fischer projection == bonds pointing down in Haworth projection
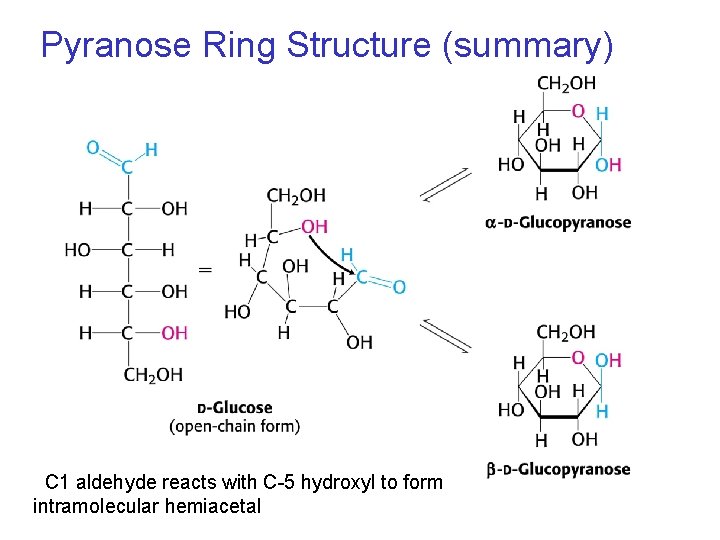
Pyranose Ring Structure (summary) C 1 aldehyde reacts with C-5 hydroxyl to form intramolecular hemiacetal
- Slides: 22