Carbohydrates Classification Monosaccharides Chiral Carbon Atoms Structures of

Carbohydrates Classification Monosaccharides Chiral Carbon Atoms Structures of Important Monosaccharides Cyclic Structures 1

Carbohydrates • Major source of energy from our diet • Composed of the elements C, H and O • Produced by photosynthesis in plants 2
Types of Carbohydrates • Monosacchrides • Disaccharides Contain 2 monosacchride units • Polysacchrides Contain many monosacchride units 3

Monosacchrides • Three Carbons = Triose • Four Carbons = Tetrose • Five Carbons = Pentose • Six Carbons = Hexose 4

Monosacchrides • Aldoses are monosacchrides with an aldehyde group and many hydroxyl (-OH) groups. • Ketoses are monosacchrides with a ketone group and many hydroxyl (-OH) groups. 5
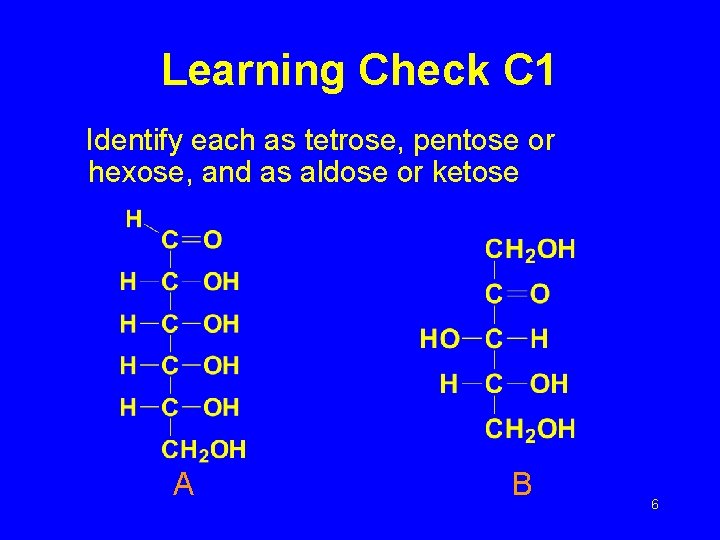
Learning Check C 1 Identify each as tetrose, pentose or hexose, and as aldose or ketose A B 6
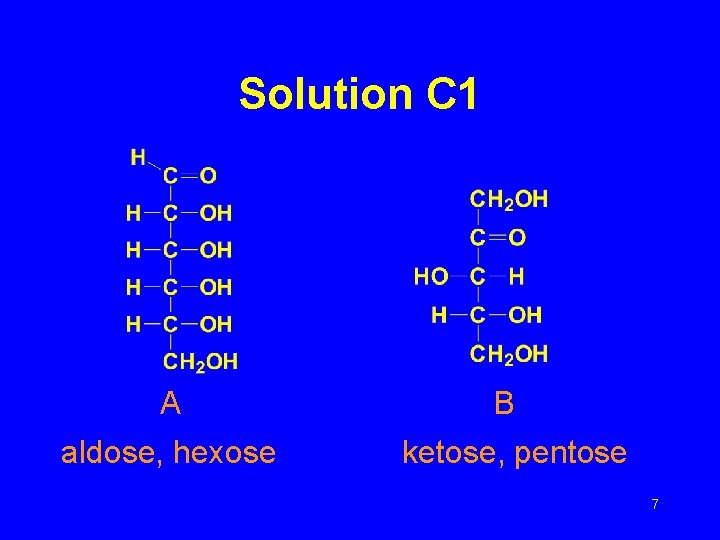
Solution C 1 A aldose, hexose B ketose, pentose 7
Chiral Objects • Chiral compounds have the same number of atoms arranged differently in space. • A chiral carbon atom has four different groups attached 8

Mirror Images • The three-dimensional structure of a chiral compound has a mirror image. • Your hands are chiral. Try to superimpose your thumbs, palms, back of hands, and little fingers. Is it possible? Why or why not? 9
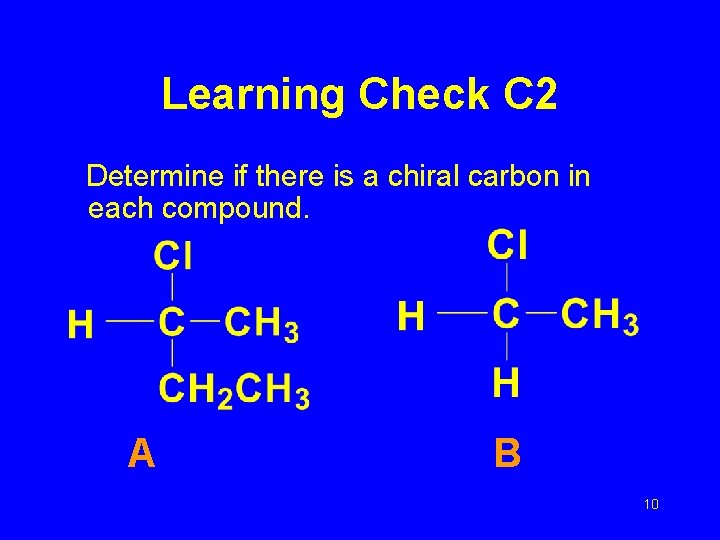
Learning Check C 2 Determine if there is a chiral carbon in each compound. A B 10
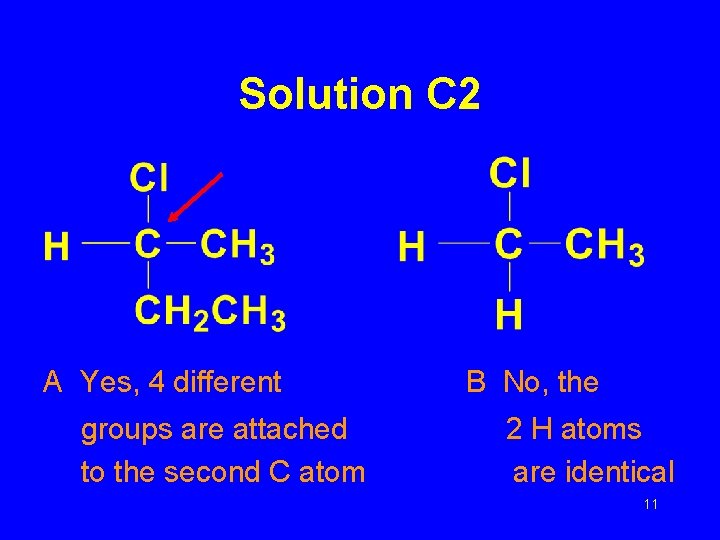
Solution C 2 A Yes, 4 different groups are attached to the second C atom B No, the 2 H atoms are identical 11

D and L Notation • D, L tells which of the two chiral isomers we are referring to. • If the –OH group on the next to the bottom carbon atom points to the right , the isomer is a D-isomer; if it points left, the isomer is L. • The D form is usually the isomer found in nature. 12
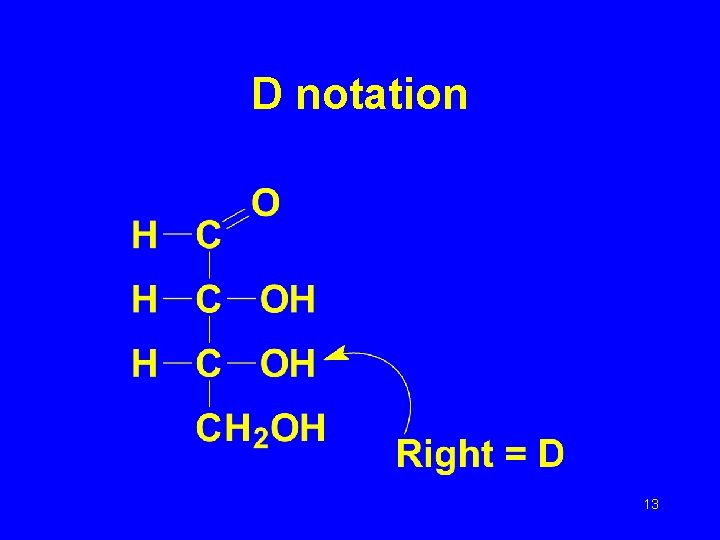
D notation 13
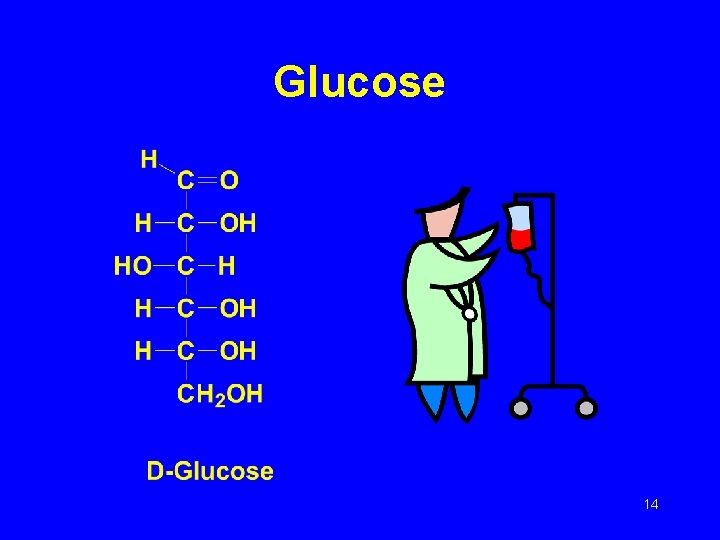
Glucose 14
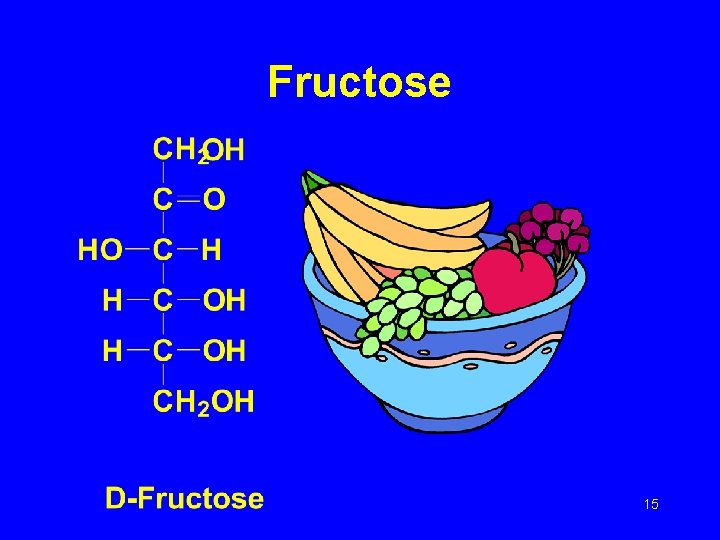
Fructose 15
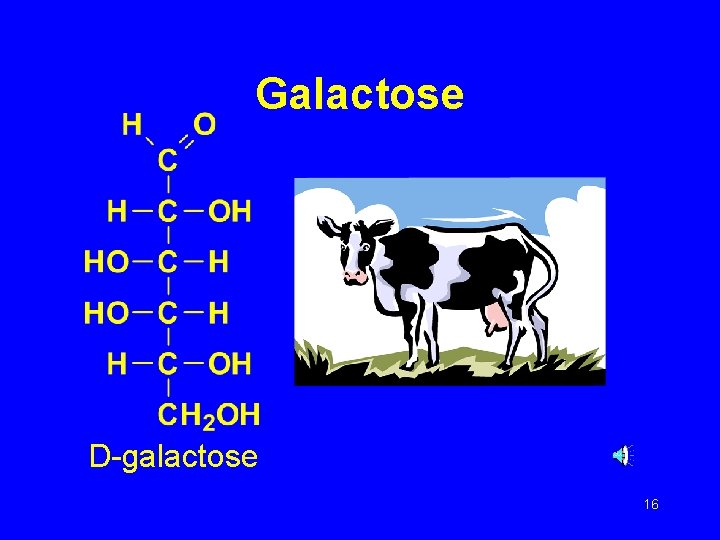
Galactose D-galactose 16
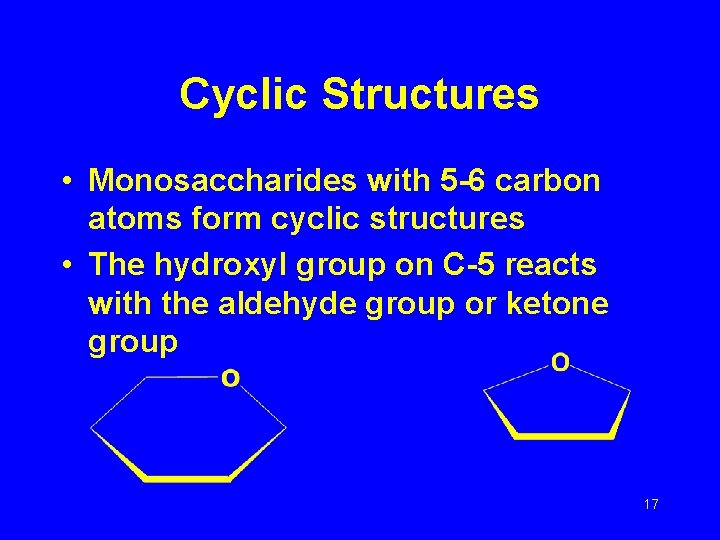
Cyclic Structures • Monosaccharides with 5 -6 carbon atoms form cyclic structures • The hydroxyl group on C-5 reacts with the aldehyde group or ketone group 17
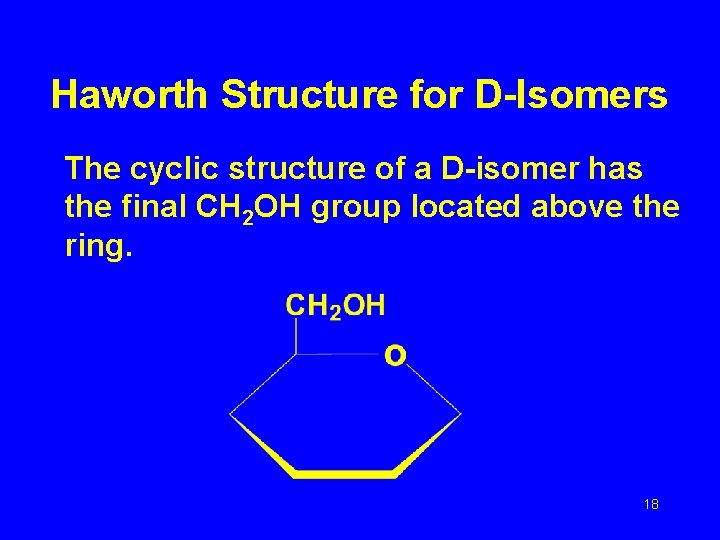
Haworth Structure for D-Isomers The cyclic structure of a D-isomer has the final CH 2 OH group located above the ring. 18

Haworth Structure for DGlucose • Write –OH groups on the right (C 2, C 4) down • Write –OH groups on the left (C 3) up • The new –OH on C 1 has two possibilites: down for anomer, up for anomer 19
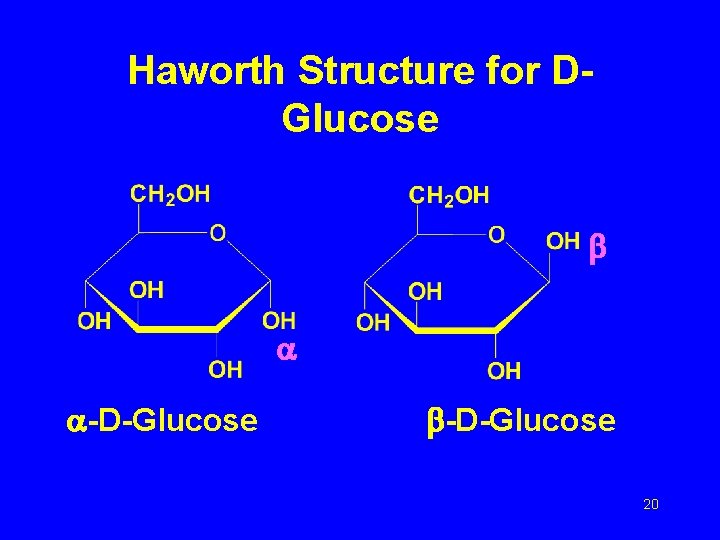
Haworth Structure for DGlucose -D-Glucose 20

Mutarotation • Mutarotation: A small amount of open chain is in equilibrium with the cyclic forms. • The most stable form of glucose is β-Dglucose. -D-glucose (36%) D-glucose (open) (trace) β-D-glucose (64%) 21
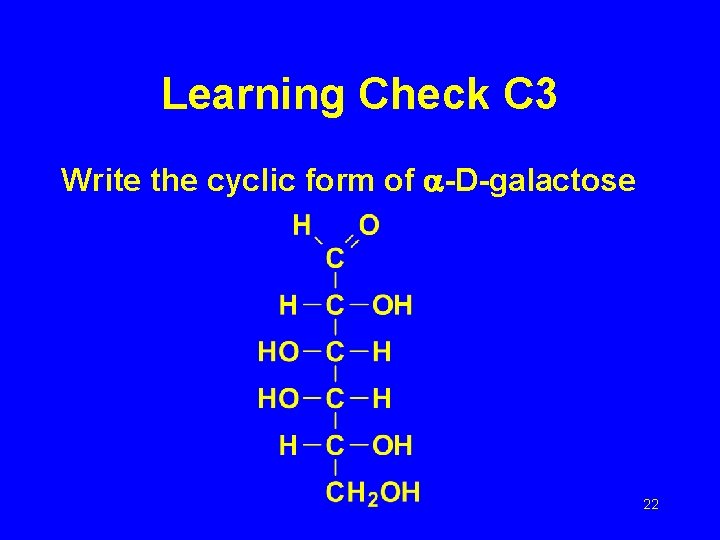
Learning Check C 3 Write the cyclic form of -D-galactose 22
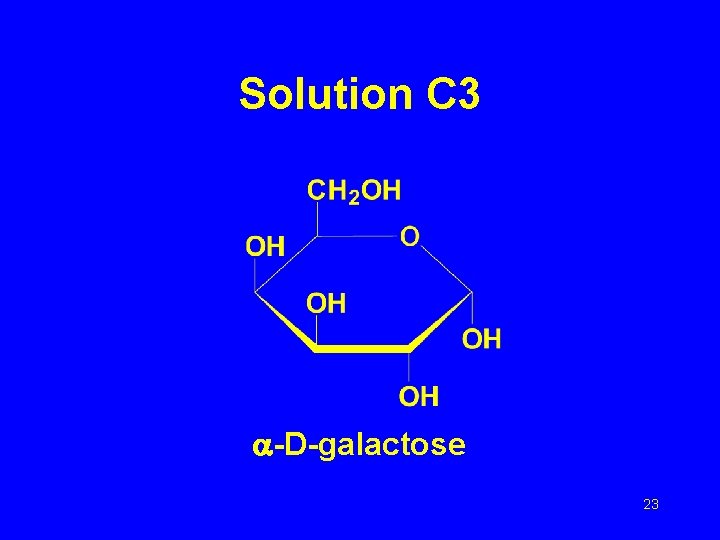
Solution C 3 -D-galactose 23
- Slides: 23