Carbohydrates and Glycobiology Carbohydrate function and classification Monosaccharides
Carbohydrates and Glycobiology • Carbohydrate function and classification • Monosaccharides – Chemical structure and properties – Linear and cyclized forms – Common monosaccharides and disaccharides • Carbohydrates can be joined to phosphates, alcohols and amines – Hexose derivatives important in biology • Polysacchardies: Glycogen, Starch, Cellulose, and Chitin
“The chemistry and biology of carbohydrates has been a Cinderella field: an area that involves much work but, alas, does not get to show off at the ball with her cousins, the genomes and proteins. ” Stella Hurtley, Robert Service, Phil Szuromi, Science Vol 291, 23 March 2001

“What has rescued this Cinderella from the shadows is no fairy godmother but a plethora of new synthetic and analytic methods that a previous generations of researchers would have found nearly magical nonetheless. ” “Glycobiology has finally become part of the mainstream”

Carbohydrates • Functions: – As energy stores and fuels – As metabolic intermediates – As part of many important molecules (ATP, ribose sugar. . ) – In polysaccharides (e. g. cell walls of bacterial and plant) – Linked to proteins and lipids (glycoconjugates) • In the extra cellular milieu, they exert effects on cellular recognition in infection, cancer, and immune response. • Carbohydrates are central to many processes that are at the core of important diseases drug design targeting a wide spectrum of diseases • Classification: mono- and polysaccharides
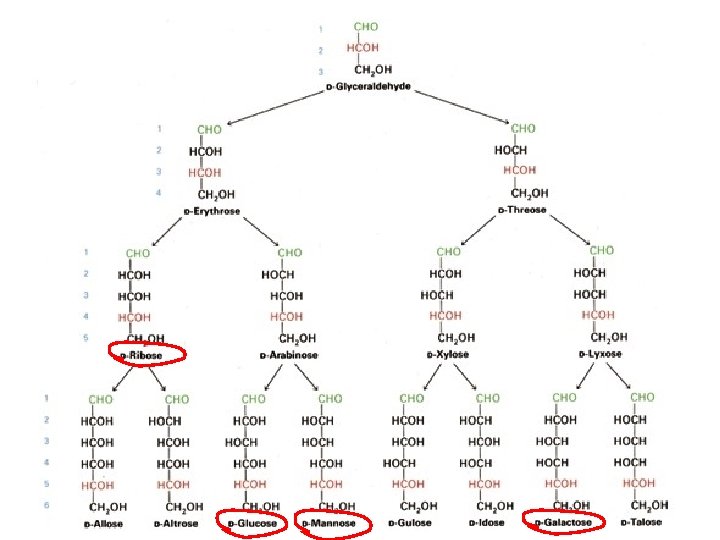
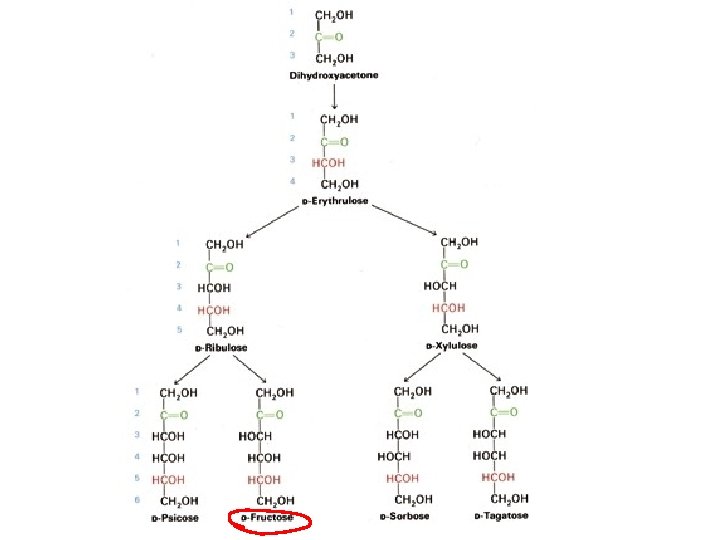
Monosaccharides • Two families of monosaccharides – Aldehydes with multiple OH groups (aldose) – Ketones with multiple OH groups (ketose) • Chemical structures of monosaccharides – Triose, tetrose, pentose, hexose, heptose • Smallest one: (CH 2 O)3 e. g. : D(L)-glyceraldehyde • Hexoses are the most common monosaccharides in nature • D-ribose and 2 -deoxy-D-ribose are components of nucleotides and nucleic acids – All except one monosaccharides have asymmetric centers • Fisher projection representation • Perspective representation
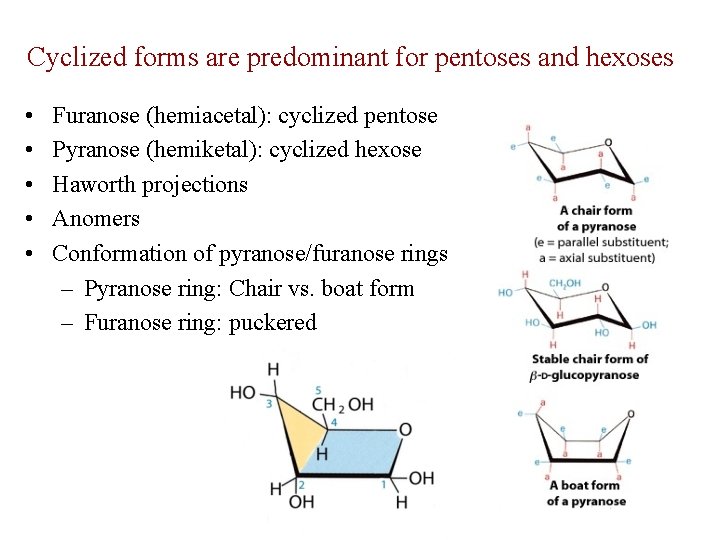
Cyclized forms are predominant for pentoses and hexoses • • • Furanose (hemiacetal): cyclized pentose Pyranose (hemiketal): cyclized hexose Haworth projections Anomers Conformation of pyranose/furanose rings – Pyranose ring: Chair vs. boat form – Furanose ring: puckered
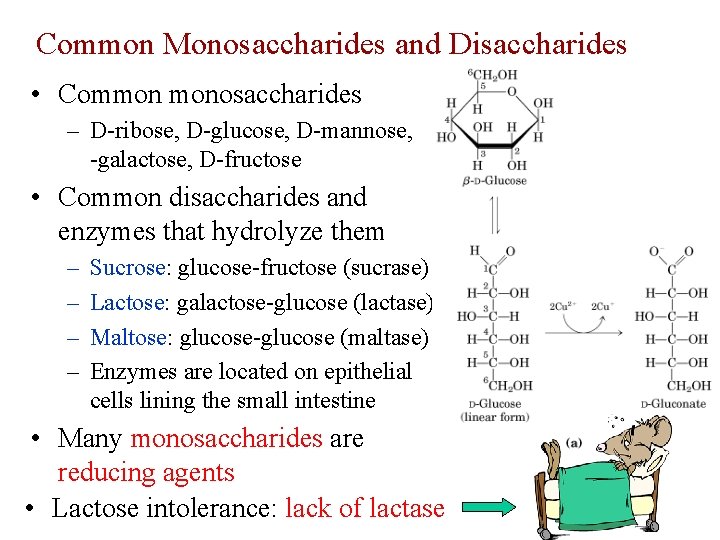
Common Monosaccharides and Disaccharides • Common monosaccharides – D-ribose, D-glucose, D-mannose, D -galactose, D-fructose • Common disaccharides and enzymes that hydrolyze them – – Sucrose: glucose-fructose (sucrase) Lactose: galactose-glucose (lactase) Maltose: glucose-glucose (maltase) Enzymes are located on epithelial cells lining the small intestine • Many monosaccharides are reducing agents • Lactose intolerance: lack of lactase

Carbohydrates Can be Joined to Phosphates, Alcohols and Amines • Sugars can be phosphorylated – Key intermediates in energy generation and biosynthesis • Carbohydrates can be joined to alcohols and amines by glycosidic bonds – N-glycosidic – O-glycosidic • Important hexose derivatives in biology (next slide)
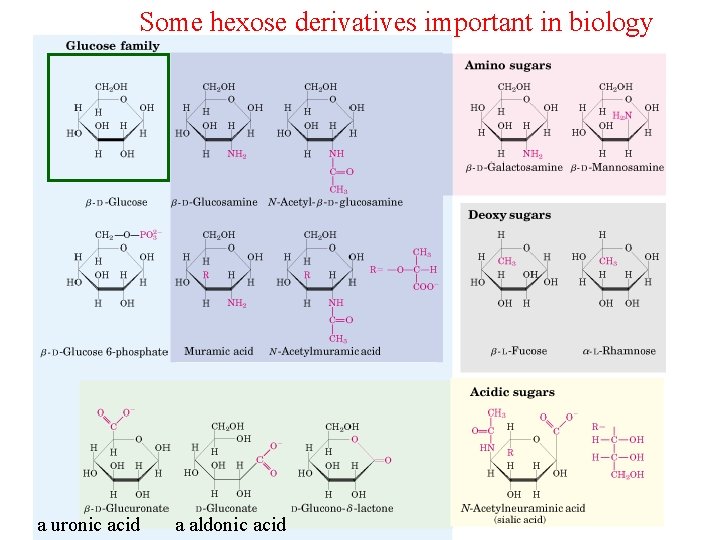
Some hexose derivatives important in biology a uronic acid a aldonic acid
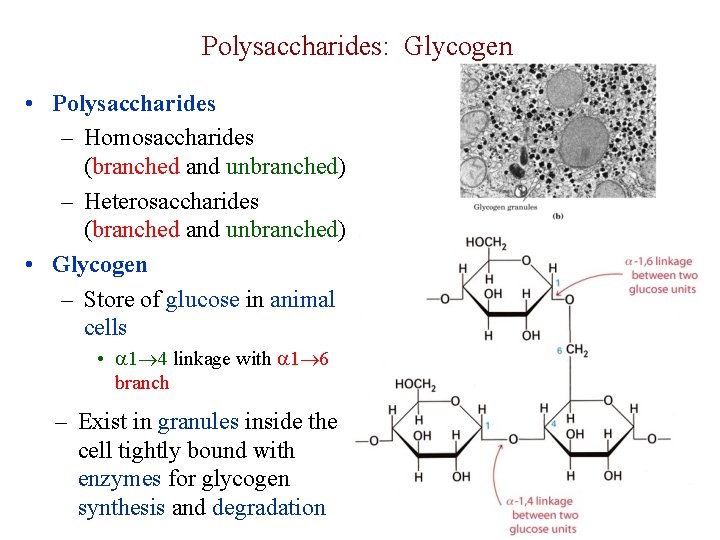
Polysaccharides: Glycogen • Polysaccharides – Homosaccharides (branched and unbranched) – Heterosaccharides (branched and unbranched) • Glycogen – Store of glucose in animal cells • 1 4 linkage with 1 6 branch – Exist in granules inside the cell tightly bound with enzymes for glycogen synthesis and degradation
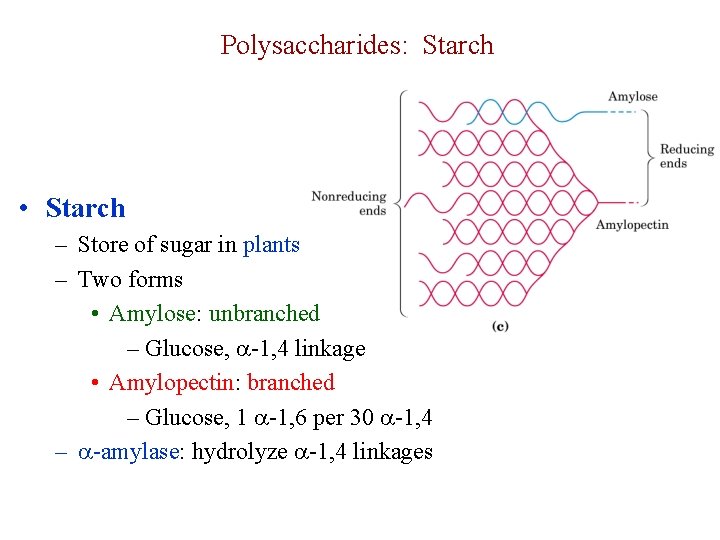
Polysaccharides: Starch • Starch – Store of sugar in plants – Two forms • Amylose: unbranched – Glucose, -1, 4 linkage • Amylopectin: branched – Glucose, 1 -1, 6 per 30 -1, 4 – -amylase: hydrolyze -1, 4 linkages
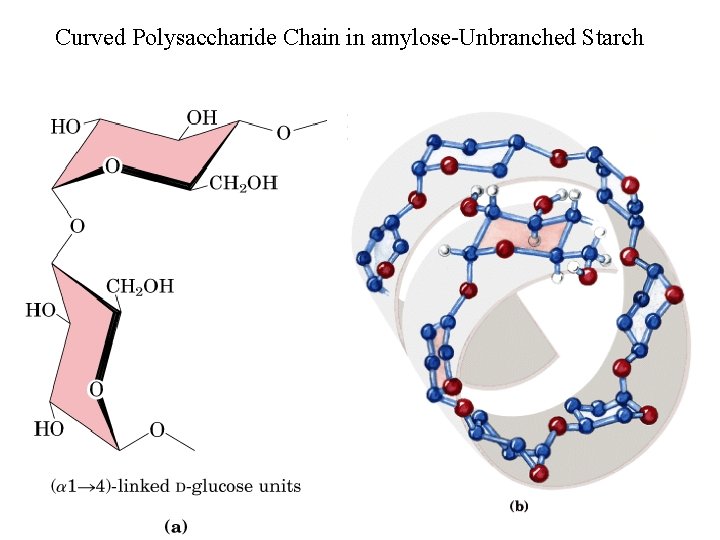
Curved Polysaccharide Chain in amylose-Unbranched Starch
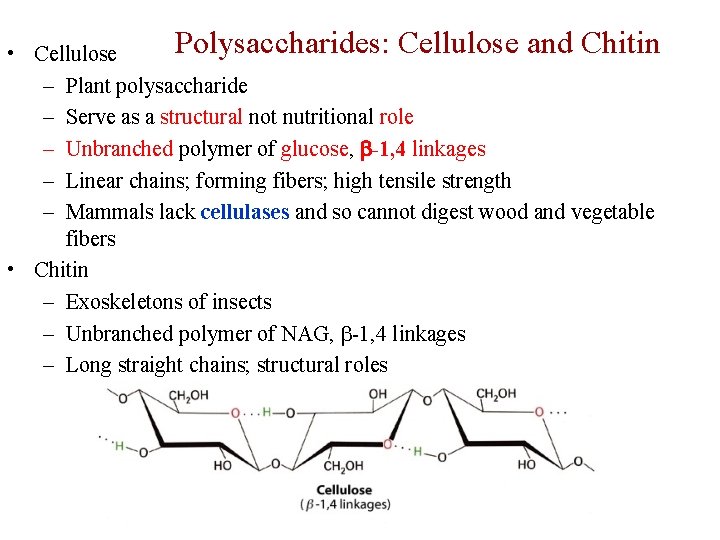
Polysaccharides: Cellulose and Chitin • Cellulose – Plant polysaccharide – Serve as a structural not nutritional role – Unbranched polymer of glucose, -1, 4 linkages – Linear chains; forming fibers; high tensile strength – Mammals lack cellulases and so cannot digest wood and vegetable fibers • Chitin – Exoskeletons of insects – Unbranched polymer of NAG, -1, 4 linkages – Long straight chains; structural roles
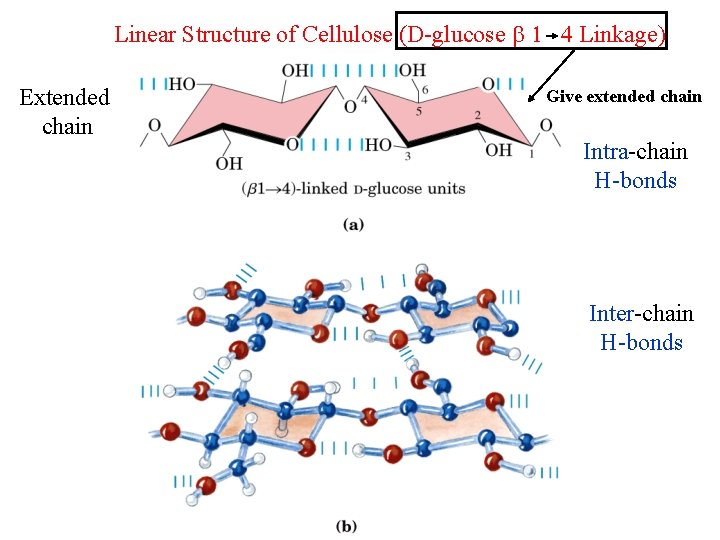
Linear Structure of Cellulose (D-glucose 1 4 Linkage) Extended chain Give extended chain Intra-chain H-bonds Inter-chain H-bonds
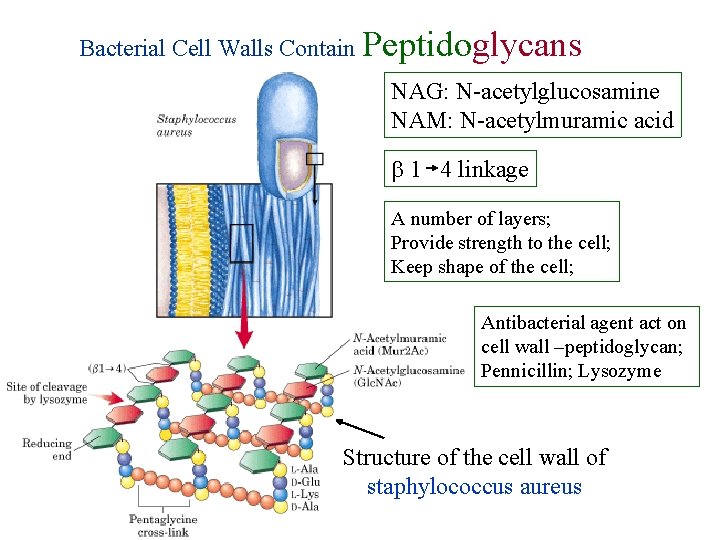
Bacterial Cell Walls Contain Peptidoglycans NAG: N-acetylglucosamine NAM: N-acetylmuramic acid 1 4 linkage A number of layers; Provide strength to the cell; Keep shape of the cell; Antibacterial agent act on cell wall –peptidoglycan; Pennicillin; Lysozyme Structure of the cell wall of staphylococcus aureus
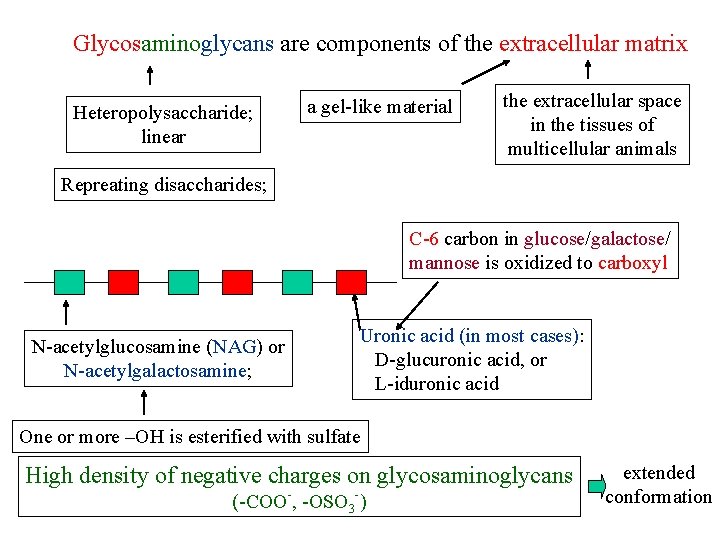
Glycosaminoglycans are components of the extracellular matrix Heteropolysaccharide; linear a gel-like material the extracellular space in the tissues of multicellular animals Repreating disaccharides; C-6 carbon in glucose/galactose/ mannose is oxidized to carboxyl N-acetylglucosamine (NAG) or N-acetylgalactosamine; Uronic acid (in most cases): D-glucuronic acid, or L-iduronic acid One or more –OH is esterified with sulfate High density of negative charges on glycosaminoglycans (-COO-, -OSO 3 -) extended conformation
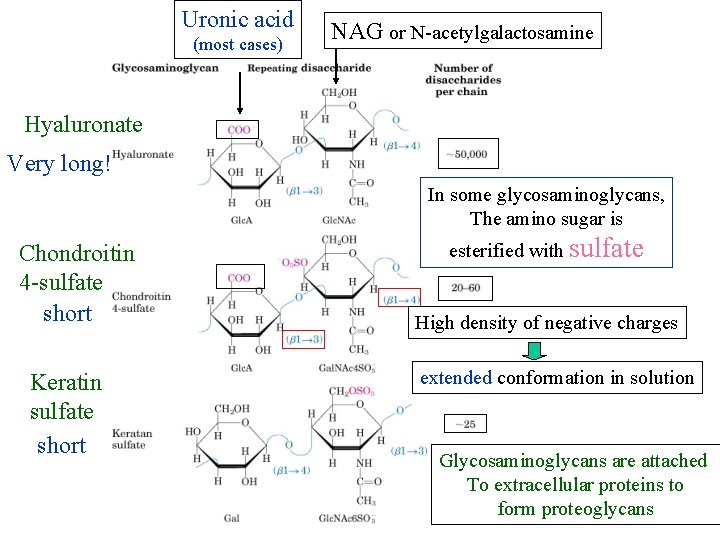
Uronic acid (most cases) NAG or N-acetylgalactosamine Hyaluronate Very long! In some glycosaminoglycans, The amino sugar is Chondroitin 4 -sulfate short Keratin sulfate short esterified with sulfate High density of negative charges extended conformation in solution Glycosaminoglycans are attached To extracellular proteins to form proteoglycans
- Slides: 19