Carbohydrate Prelab Notes Organic compound a substance containing
Carbohydrate Pre-lab Notes

Organic compound • a substance containing both Carbon (C) AND Hydrogen (H). • Examples: carbohydrates (glucose, starch), proteins, nucleic acids (DNA and RNA), and lipids.

Inorganic compound • a substance that does not contain Carbon (C) AND Hydrogen (H). • Examples: CO 2, H 2 O, O 2

Carbohydrates: • an organic molecule composed of carbon, hydrogen and oxygen • Ratio of hydrogens to oxygen 2 : 1 – Twice as many H’s or Half as many O’s • The ending –ose means carbohydrate (sugar)! • Uses: energy(used to make ATP through cellular respiration), structure (cellulose in plants and chitin in insects) • Energy is stored in the bonds of the molecule

Saccharide = sugar • Monosaccharide: means one sugar • Disaccharide means two sugars • Polysaccharide means many sugars • Examples: glucose, fructose, galactose, cellulose, sucrose, starch, lactose, amylose – Notice how they all (except starch) end in –ose? !!
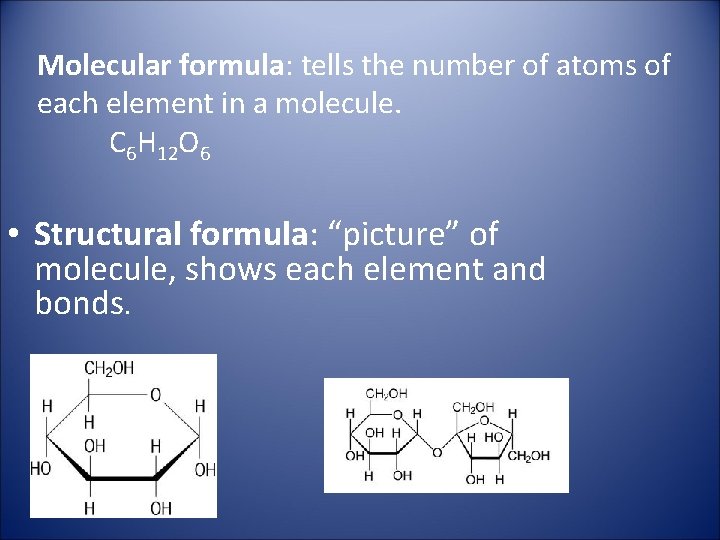
Molecular formula: tells the number of atoms of each element in a molecule. C 6 H 12 O 6 • Structural formula: “picture” of molecule, shows each element and bonds.

• Dehydration synthesis: putting together by removing water – Dehydrate = lose water – Synthesis = to make • Hydrolysis: breaking apart by adding water – Hydro = water – Lysis = break apart
- Slides: 7