Carbo QC Lab and atline Beverage Carbonation Meter

Carbo. QC Lab and at-line Beverage Carbonation Meter Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
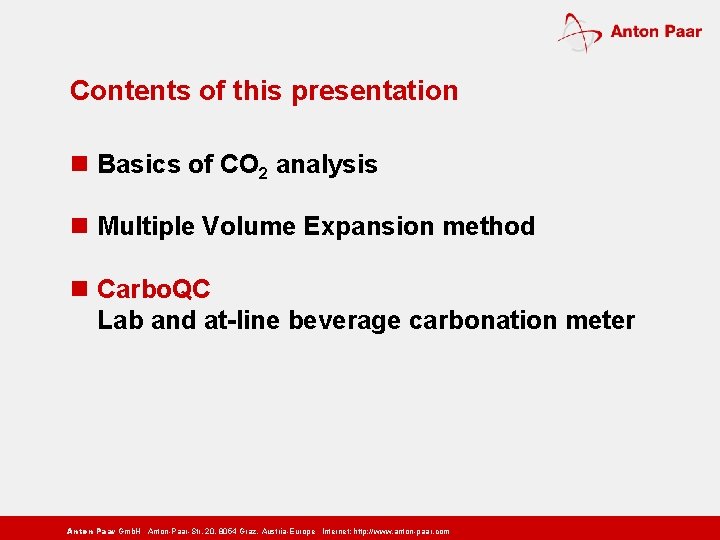
Contents of this presentation n Basics of CO 2 analysis n Multiple Volume Expansion method n Carbo. QC Lab and at-line beverage carbonation meter Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
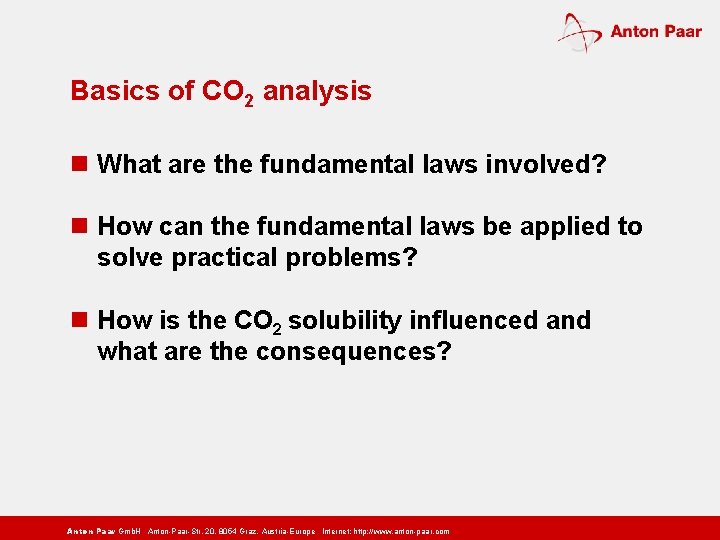
Basics of CO 2 analysis n What are the fundamental laws involved? n How can the fundamental laws be applied to solve practical problems? n How is the CO 2 solubility influenced and what are the consequences? Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
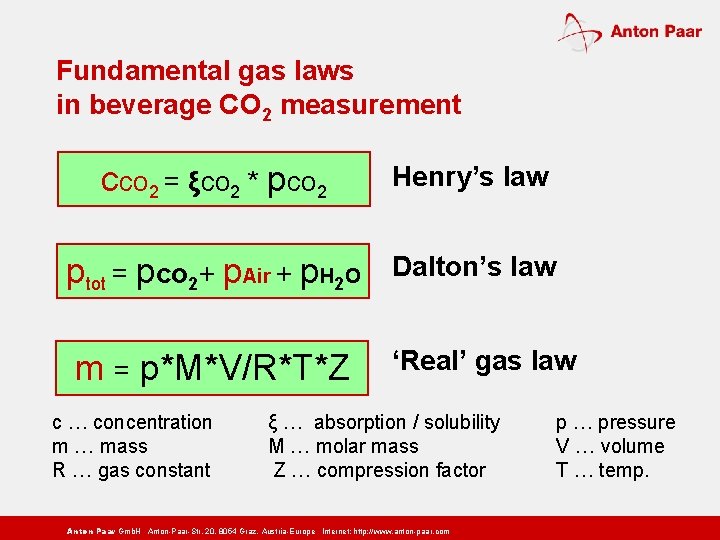
Fundamental gas laws in beverage CO 2 measurement cco 2 = ξco 2 * pco 2 Henry’s law ptot = pco 2+ p. Air + p. H 2 O Dalton’s law m = p*M*V/R*T*Z ‘Real’ gas law c … concentration m … mass R … gas constant ξ … absorption / solubility M … molar mass Z … compression factor Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com p … pressure V … volume T … temp.
![CO 2 measurement units [g/l] Volumes [Vol. ] 1 Vol. = 1. 976 g/l CO 2 measurement units [g/l] Volumes [Vol. ] 1 Vol. = 1. 976 g/l](http://slidetodoc.com/presentation_image_h/0f9888f5e255c480a0f22c01fdb918db/image-5.jpg)
CO 2 measurement units [g/l] Volumes [Vol. ] 1 Vol. = 1. 976 g/l Grams of CO 2 per liter of liquid Volumes of CO 2 at 0°C and 1 atm diss. per liquid volume Conversion Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
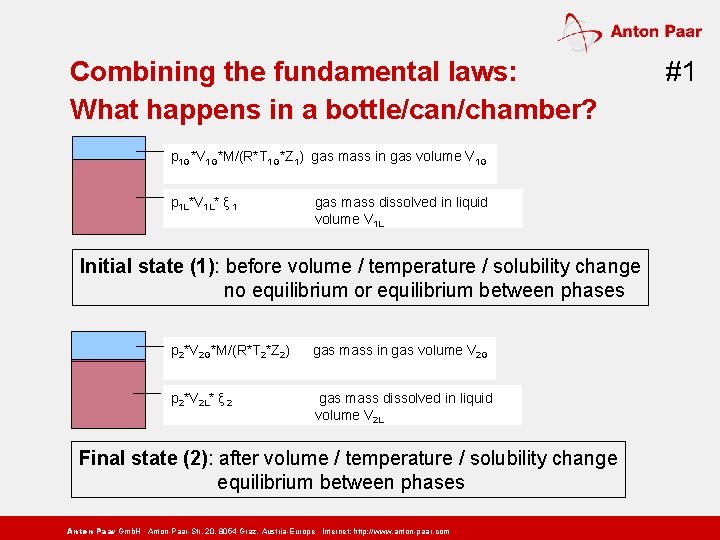
Combining the fundamental laws: What happens in a bottle/can/chamber? p 1 G*V 1 G*M/(R*T 1 G*Z 1) gas mass in gas volume V 1 G p 1 L*V 1 L* ξ 1 gas mass dissolved in liquid volume V 1 L Initial state (1): before volume / temperature / solubility change no equilibrium or equilibrium between phases p 2*V 2 G*M/(R*T 2*Z 2) gas mass in gas volume V 2 G p 2*V 2 L* ξ 2 gas mass dissolved in liquid volume V 2 L Final state (2): after volume / temperature / solubility change equilibrium between phases Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com #1
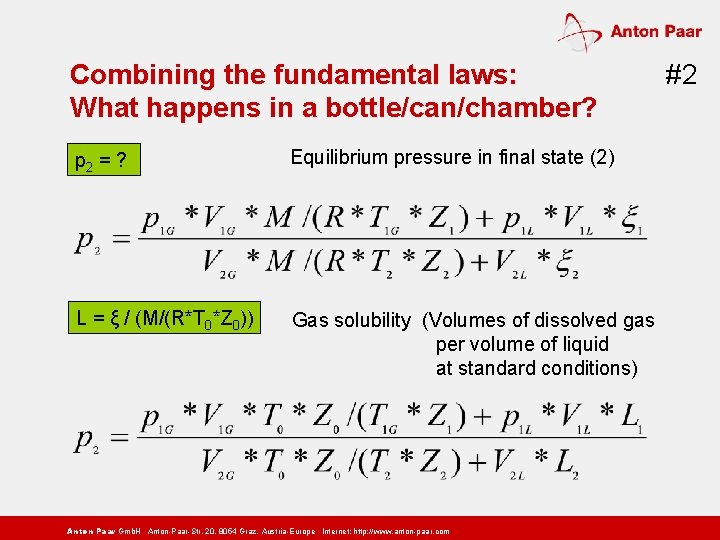
Combining the fundamental laws: What happens in a bottle/can/chamber? p 2 = ? Equilibrium pressure in final state (2) L = ξ / (M/(R*T 0*Z 0)) Gas solubility (Volumes of dissolved gas per volume of liquid at standard conditions) Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com #2
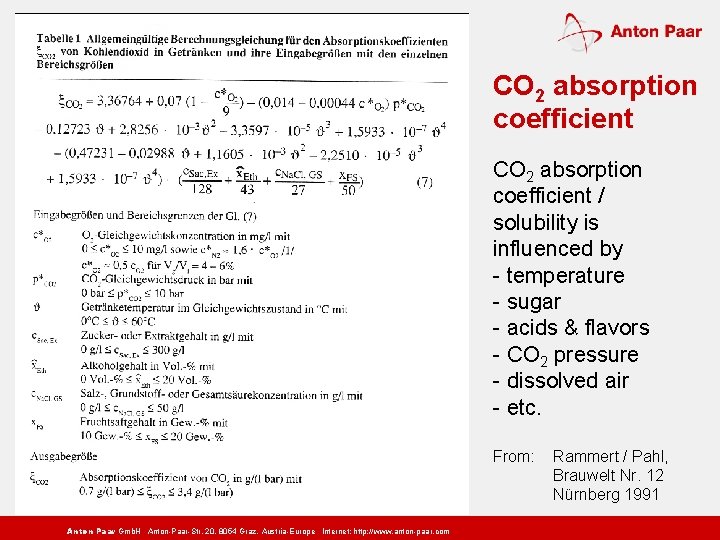
CO 2 absorption coefficient / solubility is influenced by - temperature - sugar - acids & flavors - CO 2 pressure - dissolved air - etc. From: Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com Rammert / Pahl, Brauwelt Nr. 12 Nürnberg 1991
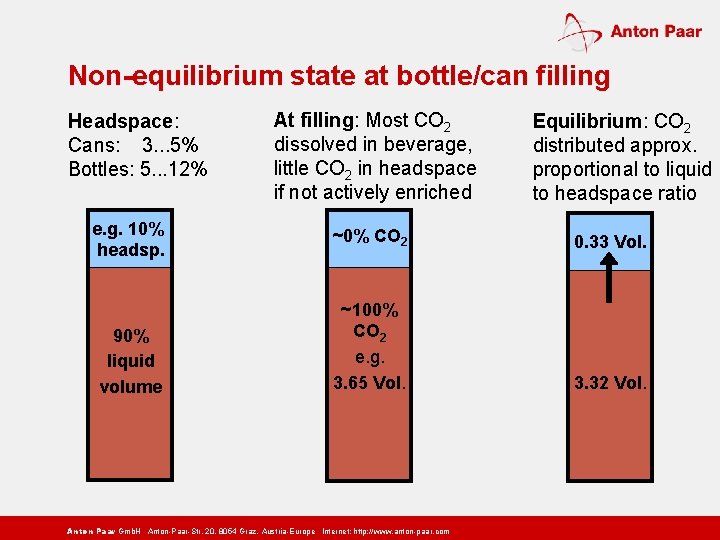
Non-equilibrium state at bottle/can filling Headspace: Cans: 3. . . 5% Bottles: 5. . . 12% e. g. 10% headsp. 90% liquid volume At filling: Most CO 2 dissolved in beverage, little CO 2 in headspace if not actively enriched Equilibrium: CO 2 distributed approx. proportional to liquid to headspace ratio ~0% CO 2 0. 33 Vol. ~100% CO 2 e. g. 3. 65 Vol. 3. 32 Vol. Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
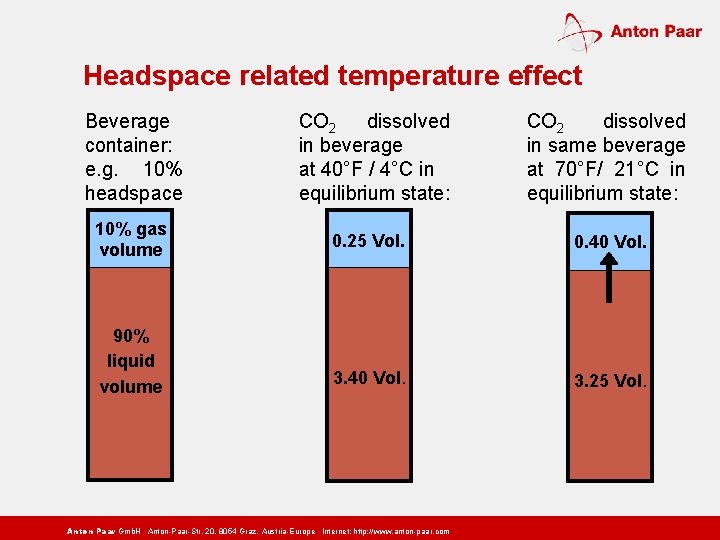
Headspace related temperature effect Beverage container: e. g. 10% headspace 10% gas volume 90% liquid volume CO 2 dissolved in beverage at 40°F / 4°C in equilibrium state: CO 2 dissolved in same beverage at 70°F/ 21°C in equilibrium state: 0. 25 Vol. 0. 40 Vol. 3. 25 Vol. Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
Multiple Volume Expansion Method n What happens to the gas partial pressures when the volume of the measuring chamber is expanded? n How and why does the Multiple Volume Expansion Method work? n What are the results? Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
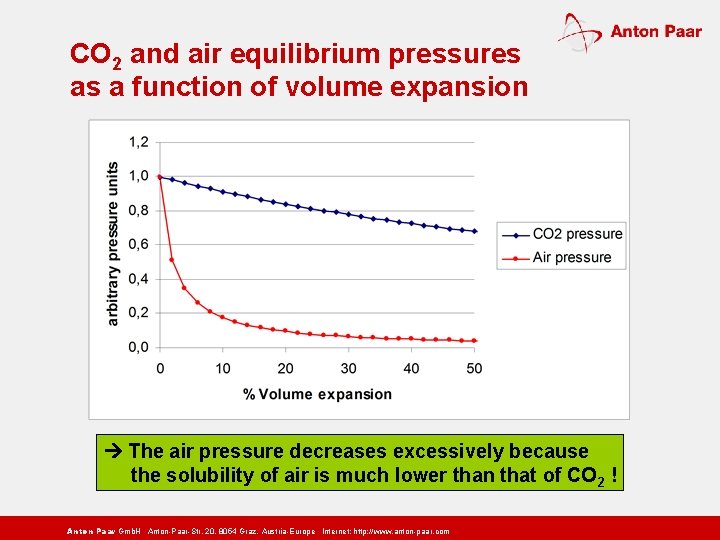
CO 2 and air equilibrium pressures as a function of volume expansion The air pressure decreases excessively because the solubility of air is much lower than that of CO 2 ! Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
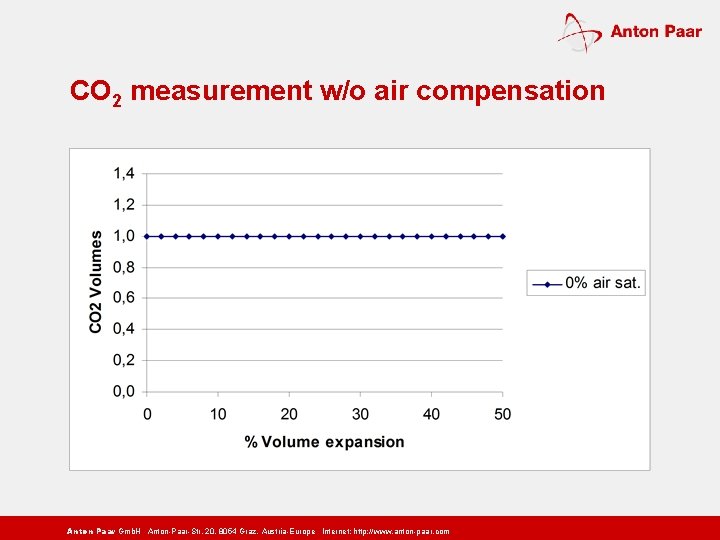
CO 2 measurement w/o air compensation Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
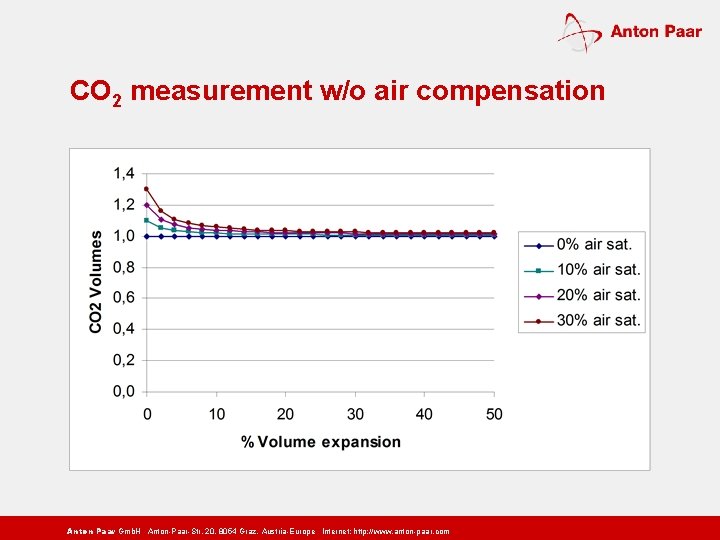
CO 2 measurement w/o air compensation Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
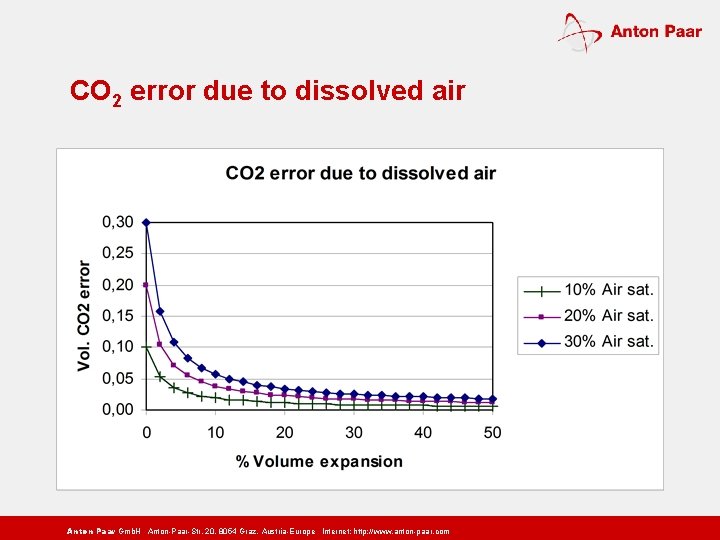
CO 2 error due to dissolved air Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

How does the Multiple Volume Expansion Method work? n A measuring chamber is completely filled with sample and sealed. n The volume of the measuring chamber is expanded. n Pressure and temperature equilibrium is generated. n Equilibrium pressure and temperature are measured. n The measuring chamber volume is further expanded, equilibrium is generated and pressure and temperature again measured. n The two pressures/temperatures are used for CO 2 determination and dissolved air compensation. Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
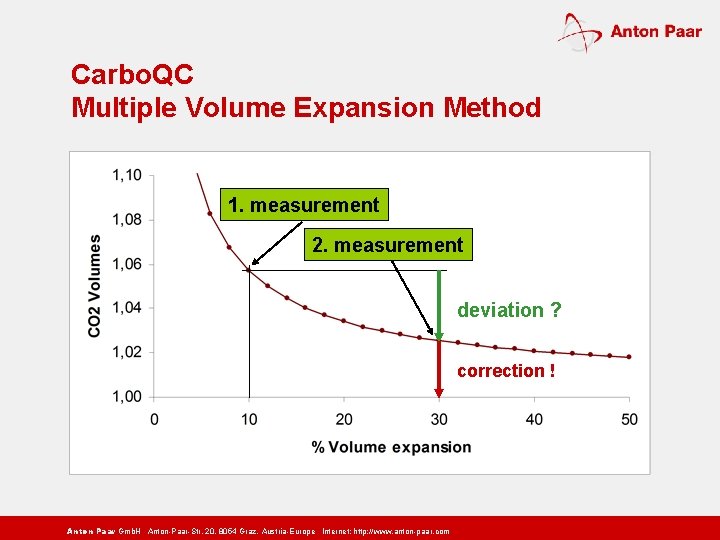
Carbo. QC Multiple Volume Expansion Method 1. measurement 2. measurement deviation ? correction ! Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
Why does the Multiple Volume Expansion Method work? n The Multiple Volume Expansion method makes use of the fact that the solubility of CO 2 is much higher than that of air. n By expanding the volume of the measuring chamber the partial pressures of CO 2 and air change very differently. n Therefore CO 2 and air partial pressures can be distinguished and the effect of dissolved air removed. Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
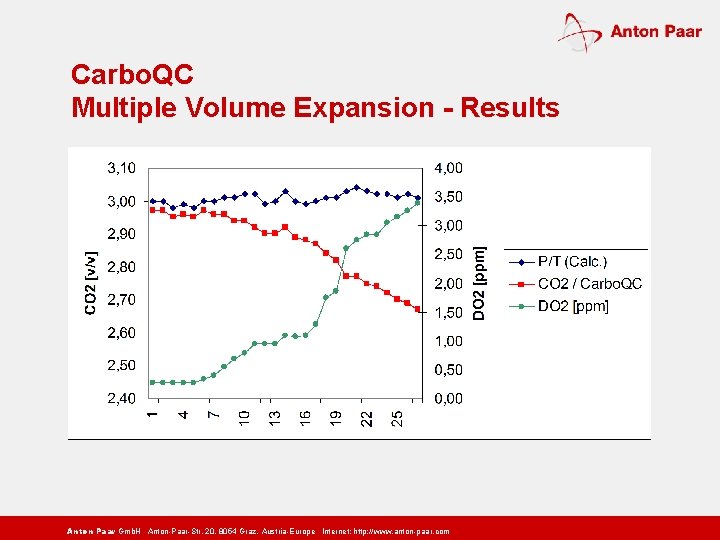
Carbo. QC Multiple Volume Expansion - Results Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Carbo. QC Lab and at-line beverage carbonation meter n New patented MVE Multiple Volume Expansion method n No influence from dissolved air n For accurate and traceable CO 2 measurements Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Carbo. QC Measuring chamber Display Keypad Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Carbo. QC Rear view Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Carbo. QC Water-tight connection plug ON / OFF switch Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Carbo. QC with semi-automatic filling device Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Specifications of the Carbo. QC Measuring range 0 to 12 g/l (0 to 6 Vol. ) at 30°C (86°F) 0 to 20 g/l (0 to 10 Vol. ) < 15°C (59°F) Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Specifications of the Carbo. QC Repeatability 0. 005 bar (0. 07 psia) 0. 05°C (0. 1°F) 0. 01 g/l (0. 005 Vol. ) Reproducibility 0. 015 bar (0. 21 psia) 0. 1°C (0. 2 °F) 0. 05 g/l (0. 025 Vol. ) Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
Benefits of the Carbo. QC n No influence from dissolved air n Highly accurate absolute pressure sensor no weather and sea level influence n Very accurate temperature measurement due to impeller technique n No calibration/adjustment with reference methods or CO 2 standards necessary n Zero point calibration simply with (tap) water n Small amounts of sample n Fast and accurate results in a wide temperature range n Versatile and easy to use with PFD filling device Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
Comparison to Zahm & Nagel results n n Zahm & Nagel requires snifting (venting) at bottles to decrease air influence leads to temperature & headspace volume dependent loss of CO 2 Zahm & Nagel is subject to full influence of air at cans due to no snifting being possible Zahm & Nagel pressure and temperature measurement provides only limited accuracy Headspace related temperature effect causes deviations àThe Carbo. QC provides more precise results than Zahm & Nagel àCarbo. QC results are lower due to lack of air influence Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
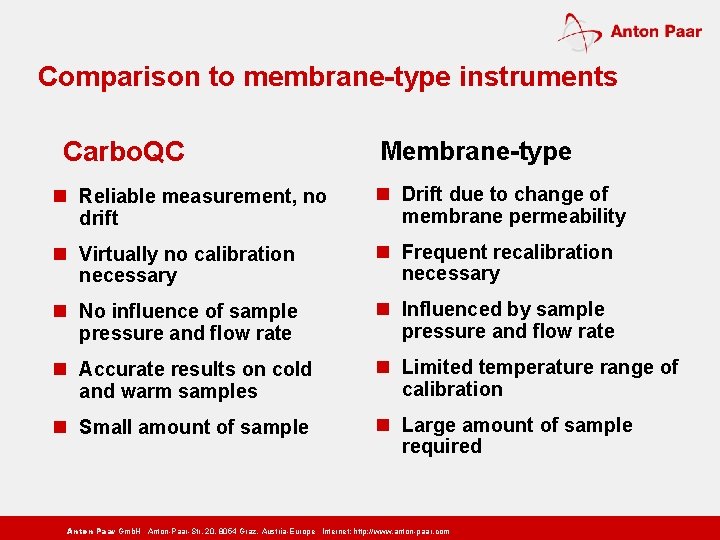
Comparison to membrane-type instruments Carbo. QC Membrane-type n Reliable measurement, no drift n Drift due to change of membrane permeability n Virtually no calibration necessary n Frequent recalibration necessary n No influence of sample pressure and flow rate n Influenced by sample pressure and flow rate n Accurate results on cold and warm samples n Limited temperature range of calibration n Small amount of sample n Large amount of sample required Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com

Carbo. QC summary n Lab and at-line beverage carbonation meter n Patented Multiple Volume Expansion method No influence from dissolved air n Easy to use, fast and accurate n Wide measurement temperature range n Small amount of sample Anton Paar Gmb. H Anton-Paar-Str. 20, 8054 Graz, Austria-Europe Internet: http: // www. anton-paar. com
- Slides: 30