CARAMELIZATION OBJECTIVES 1 Types of caramel produced 2

CARAMELIZATION OBJECTIVES 1. Types of caramel produced; 2. Mechanism and major chemical reactions; 3. Caramel usage.
CARAMELIZATION 1 1. Significance – about 11% of all colorants used; around 200 000 tons annual consumption 1850 г. – used for the first time in Europe commercially According to the FDA, “The color additive caramel is the dark-brown liquid or solid material resulting from the carefully controlled heat treatment of the following food-grade carbohydrates: dextrose, invert sugar, lactose, malt syrup, molasses, starch hydrolysates and fractions thereof, sucrose. ” 36 According to JECFA (The Joint FAO/WHO Expert Committee on Food Additives), “Caramel is a complex mixture of compounds, some of which are in the form of colloidal aggregates, manufactured by heating carbohydrates either alone or in the presence of food-grade acids, alkalis or salts; classified according to the reactant used. ” 41 36. U. S. Food & Drug Administration, Summary of Color Additives Listed for Use in the United States in Food, Drugs, Cosmetics and Medical Devices, Washington, D. C. , 1999. 41. JECFA, Combined compendium of food additive specifications, http: //www. fao. org.
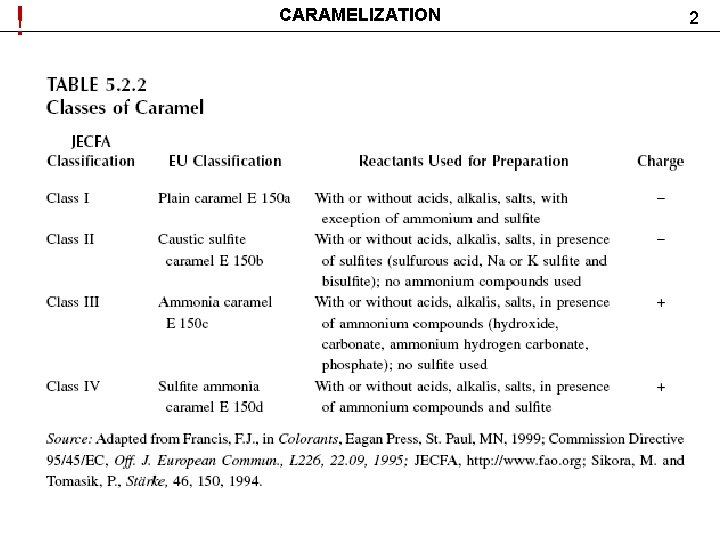
! CARAMELIZATION 2

CARAMELIZATION 2. Obtaining; phases Raw materials – glucose, glucose-fructose syrup (HFCS), sugar, hydrolysates – corn, wheat, tapioca etc. Chemical reactions involved – hydrolysis, enolization, dehydration, bonds (С-С) rupture, aldolization, cyclization, radical reactions Requirements Hue index – 3. 5 -7. 5 Hue index = 10 log (A 510 / A 610) Initial caramelization temperatures of common carbohydrates Sugar Temperature Fructose 110° C Galactose 160° C Glucose 160° C Maltose 180° C Saccharose 160° C 3
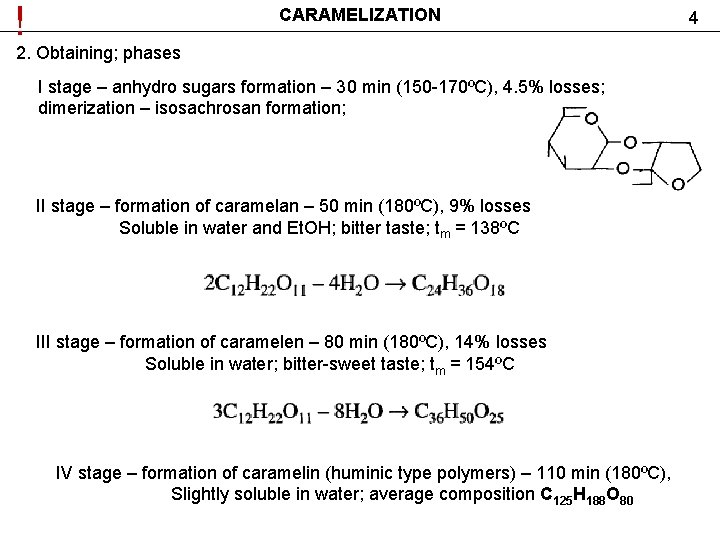
! CARAMELIZATION 2. Obtaining; phases I stage – anhydro sugars formation – 30 min (150 -170ºС), 4. 5% losses; dimerization – isosachrosan formation; II stage – formation of caramelan – 50 min (180ºС), 9% losses Soluble in water and Et. OH; bitter taste; tm = 138ºС III stage – formation of caramelen – 80 min (180ºС), 14% losses Soluble in water; bitter-sweet taste; tm = 154ºС IV stage – formation of caramelin (huminic type polymers) – 110 min (180ºС), Slightly soluble in water; average composition С 125 Н 188 О 80 4
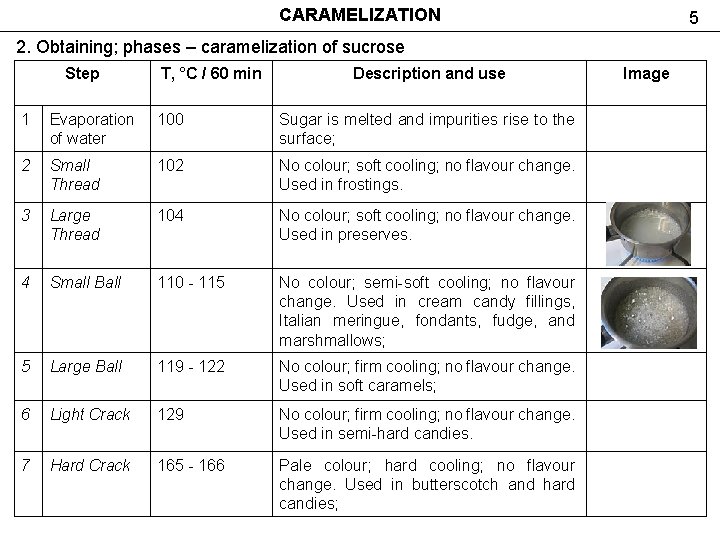
CARAMELIZATION 5 2. Obtaining; phases – caramelization of sucrose Step T, °C / 60 min Description and use 1 Evaporation of water 100 Sugar is melted and impurities rise to the surface; 2 Small Thread 102 No colour; soft cooling; no flavour change. Used in frostings. 3 Large Thread 104 No colour; soft cooling; no flavour change. Used in preserves. 4 Small Ball 110 - 115 No colour; semi-soft cooling; no flavour change. Used in cream candy fillings, Italian meringue, fondants, fudge, and marshmallows; 5 Large Ball 119 - 122 No colour; firm cooling; no flavour change. Used in soft caramels; 6 Light Crack 129 No colour; firm cooling; no flavour change. Used in semi-hard candies. 7 Hard Crack 165 - 166 Pale colour; hard cooling; no flavour change. Used in butterscotch and hard candies; Image

CARAMELIZATION 2. Obtaining; phases – caramelization of sucrose 8 Extra-hard Crack 168 Slight colour; shatters like glass during cooling; no flavour change. Used in hard candies; 9 Light Carmel 180 Pale amber to golden brown; rich flavour. 10 Medium Carmel 180 - 188 Golden brown to chestnut brown; rich flavour; 11 Dark Carmel 188 - 204 Very dark and bitter; smells burned. Used for colouring, but lack of appropriate sweetness; 12 Black Jack 210 Also known as "monkey's blood. " At this point, the sugar begins to breaks down to pure carbon. Burning flavour. 6
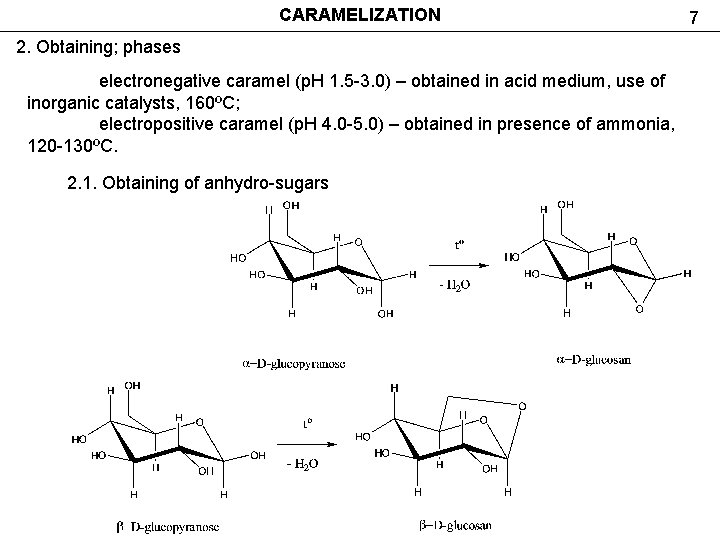
CARAMELIZATION 2. Obtaining; phases electronegative caramel (р. Н 1. 5 -3. 0) – obtained in acid medium, use of inorganic catalysts, 160ºС; electropositive caramel (р. Н 4. 0 -5. 0) – obtained in presence of ammonia, 120 -130ºС. 2. 1. Obtaining of anhydro-sugars 7

CARAMELIZATION 2. Obtaining; stages 2. 1. Obtaining of anhydro sugars 8
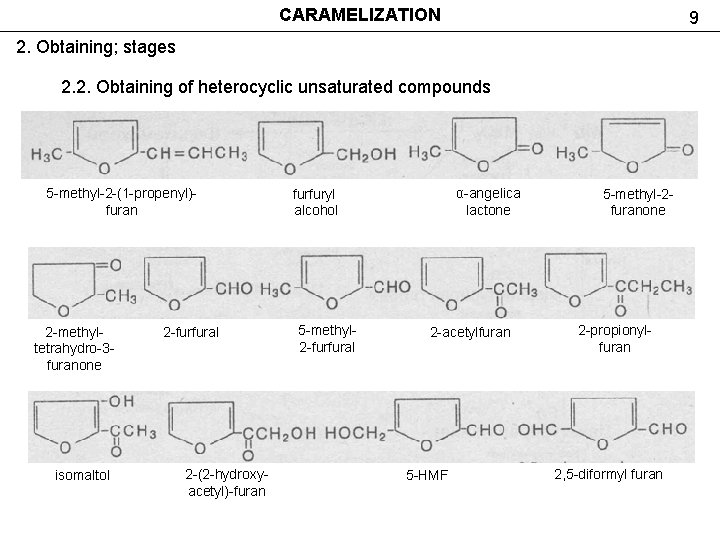
CARAMELIZATION 9 2. Obtaining; stages 2. 2. Obtaining of heterocyclic unsaturated compounds 5 -methyl-2 -(1 -propenyl)furan 2 -methyltetrahydro-3 furanone isomaltol 2 -furfural 2 -(2 -hydroxyacetyl)-furan α-angelica lactone furfuryl alcohol 5 -methyl 2 -furfural 2 -acetylfuran 5 -HMF 5 -methyl-2 furanone 2 -propionylfuran 2, 5 -diformyl furan
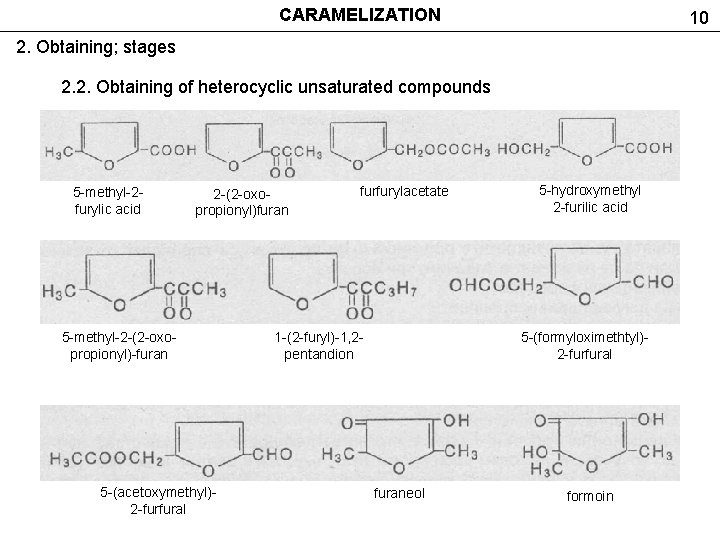
CARAMELIZATION 10 2. Obtaining; stages 2. 2. Obtaining of heterocyclic unsaturated compounds 5 -methyl-2 furylic acid 2 -(2 -oxopropionyl)furan 5 -methyl-2 -(2 -oxopropionyl)-furan 5 -(acetoxymethyl)2 -furfural furfurylacetate 1 -(2 -furyl)-1, 2 pentandion 5 -hydroxymethyl 2 -furilic acid 5 -(formyloximethtyl)2 -furfural furaneol formoin
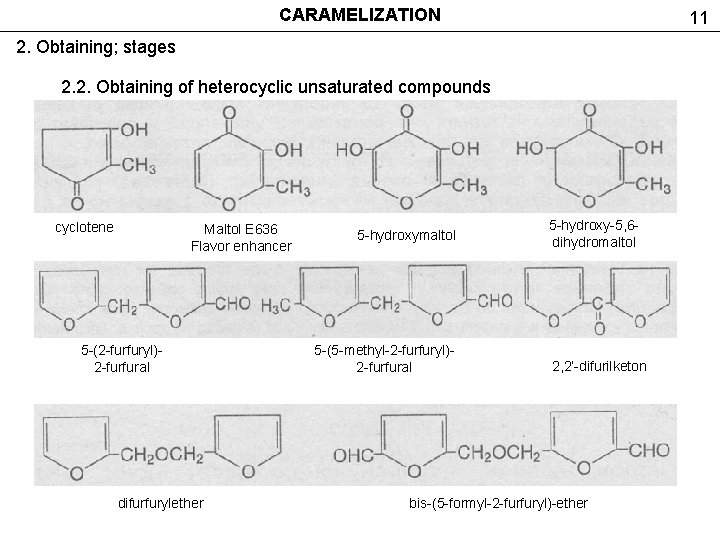
CARAMELIZATION 11 2. Obtaining; stages 2. 2. Obtaining of heterocyclic unsaturated compounds cyclotene Maltol E 636 Flavor enhancer 5 -(2 -furfuryl)2 -furfural difurfurylether 5 -hydroxymaltol 5 -(5 -methyl-2 -furfuryl)2 -furfural 5 -hydroxy-5, 6 dihydromaltol 2, 2’-difurilketon bis-(5 -formyl-2 -furfuryl)-ether
CARAMELIZATION 2. Obtaining; stages Some flavors present in the caramels diacetyl (buttery flavor); esters, lactones – rum flavor, sweetish furans – roasted nuts flavor maltol and isomaltol – caramel-like, sweetish, burnt (well-done) 12
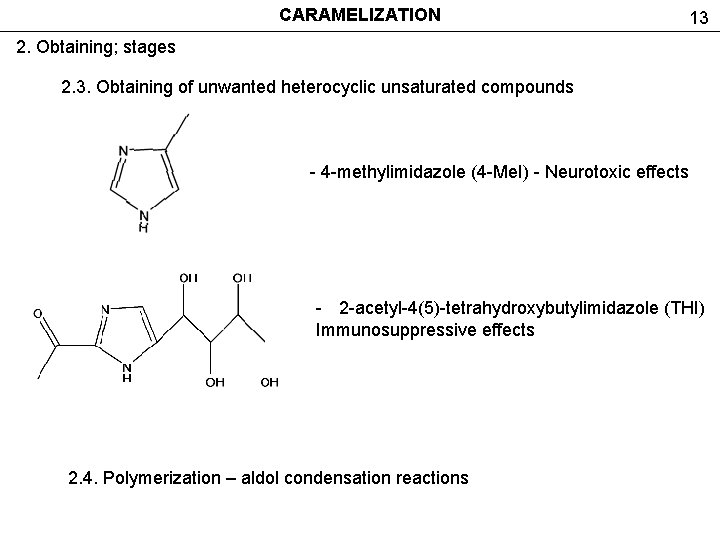
CARAMELIZATION 13 2. Obtaining; stages 2. 3. Obtaining of unwanted heterocyclic unsaturated compounds - 4 -methylimidazole (4 -Me. I) - Neurotoxic effects - 2 -acetyl-4(5)-tetrahydroxybutylimidazole (THI) Immunosuppressive effects 2. 4. Polymerization – aldol condensation reactions
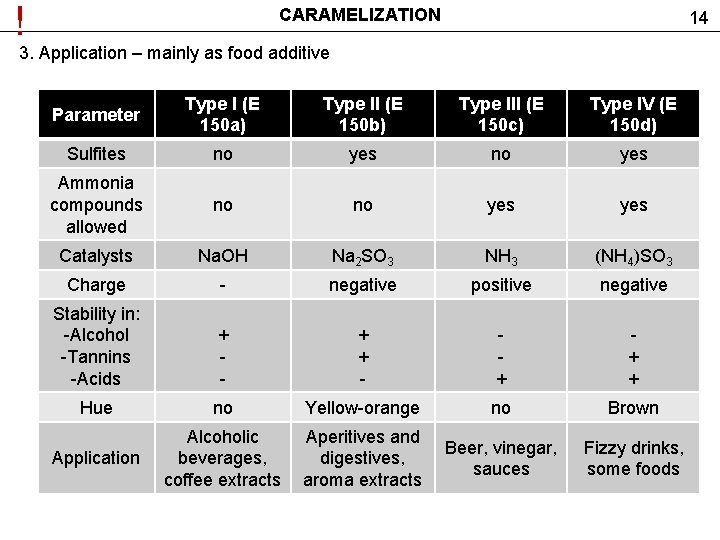
! CARAMELIZATION 14 3. Application – mainly as food additive Parameter Type I (E 150 a) Type II (E 150 b) Type III (E 150 c) Type IV (E 150 d) Sulfites no yes Ammonia compounds allowed no no yes Catalysts Na. OH Na 2 SO 3 NH 3 (NH 4)SO 3 Charge - negative positive negative Stability in: -Alcohol -Tannins -Acids + - + + + Hue no Yellow-orange no Brown Application Alcoholic beverages, coffee extracts Aperitives and digestives, aroma extracts Beer, vinegar, sauces Fizzy drinks, some foods
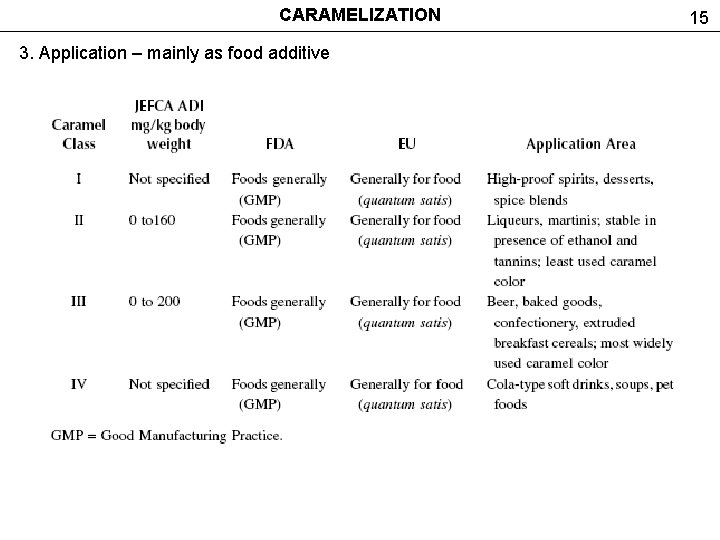
CARAMELIZATION 3. Application – mainly as food additive 15
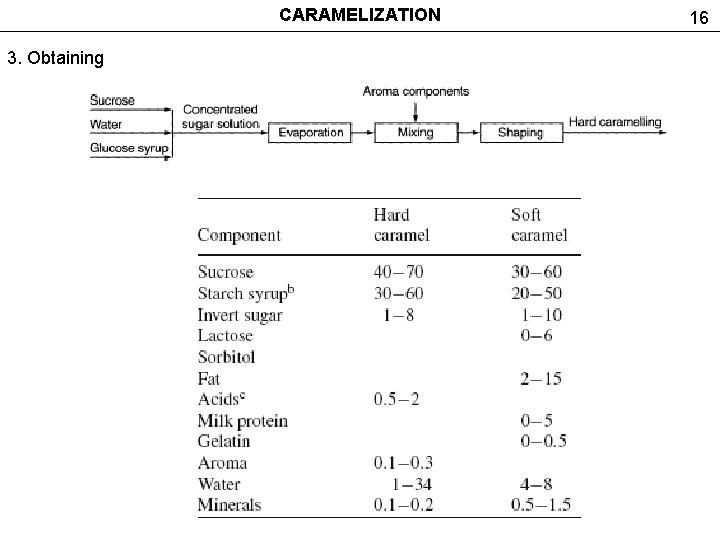
CARAMELIZATION 3. Obtaining 16

CARAMELIZATION Interactions with other substances Over 700 ppm – could lead to aspartame hydrolysis. Adulteration of juices, coffee, honey. Caramelization could be regarded as part of the Maillard reaction and as separate reaction. Info - http: //www. food-info. net/uk/colour/caramel. htm 17
- Slides: 18