Capillary Phenomena 5 Laws of Interfacial Engineering Ger

- Slides: 18

Capillary Phenomena 5 Laws of Interfacial Engineering Ger Koper Interfacial Engineering, 5 Laws

Introduction Dispersions are often not (thermodynamically) stable Solvent quality Surface free energy • Solubility parameters • Coarsening: minimize area • Polarity (if dissociated) • 5 Laws of Interfacial Engineering • Despite entropy gain upon dispersion 01/03/2021 Interfacial Engineering, 5 Laws 2

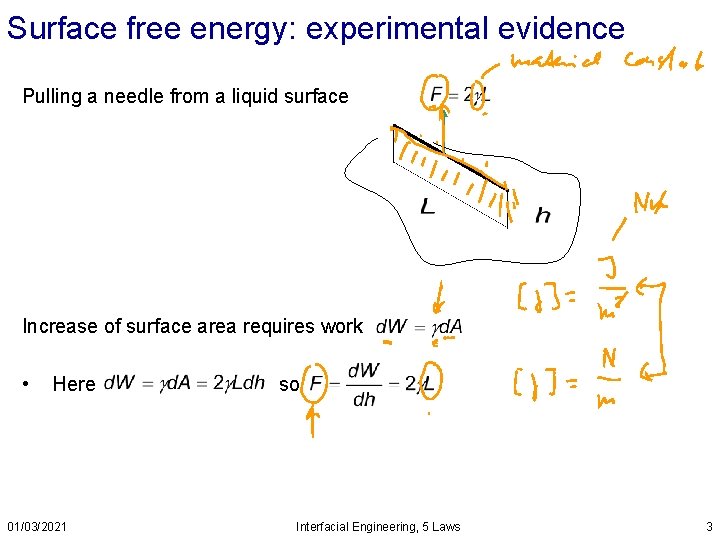
Surface free energy: experimental evidence Pulling a needle from a liquid surface Increase of surface area requires work • Here 01/03/2021 so Interfacial Engineering, 5 Laws 3

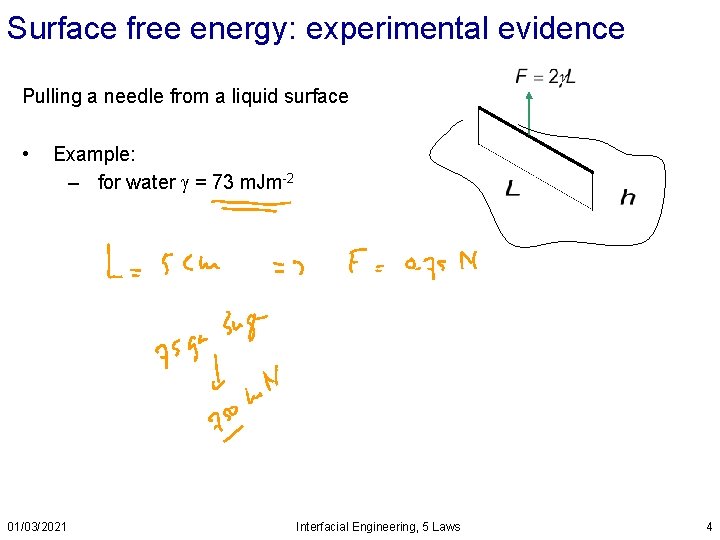
Surface free energy: experimental evidence Pulling a needle from a liquid surface • Example: – for water = 73 m. Jm-2 01/03/2021 Interfacial Engineering, 5 Laws 4

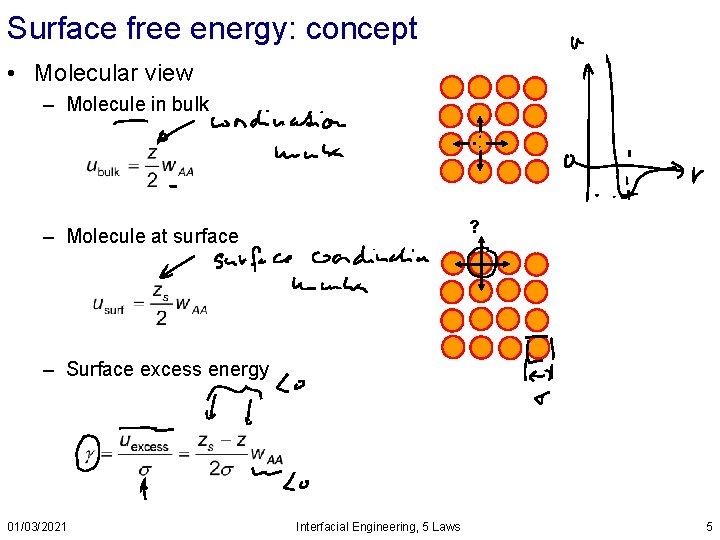
Surface free energy: concept • Molecular view – Molecule in bulk ? – Molecule at surface – Surface excess energy 01/03/2021 Interfacial Engineering, 5 Laws 5

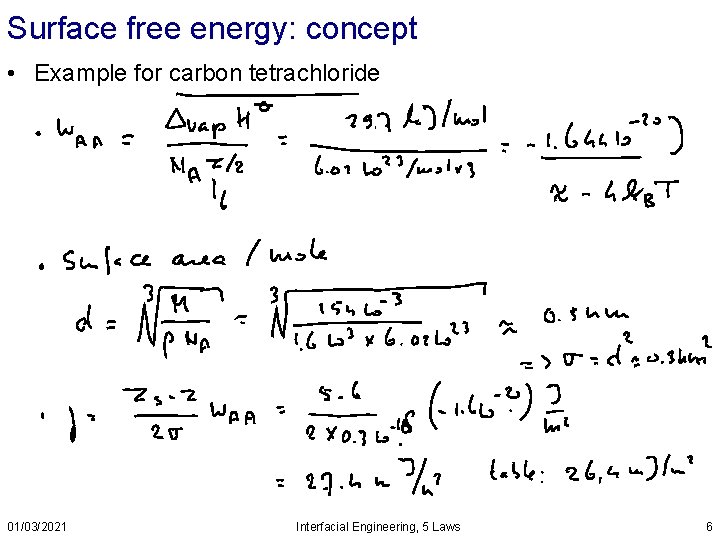
Surface free energy: concept • Example for carbon tetrachloride 01/03/2021 Interfacial Engineering, 5 Laws 6

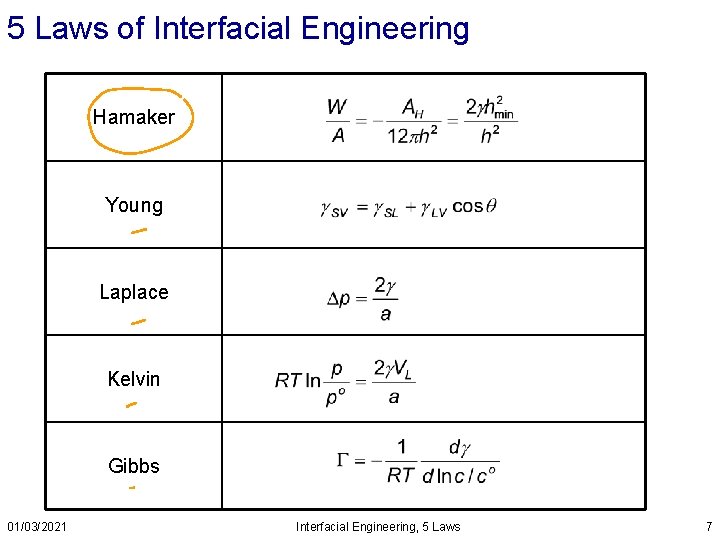
5 Laws of Interfacial Engineering Hamaker Young Laplace Kelvin Gibbs 01/03/2021 Interfacial Engineering, 5 Laws 7

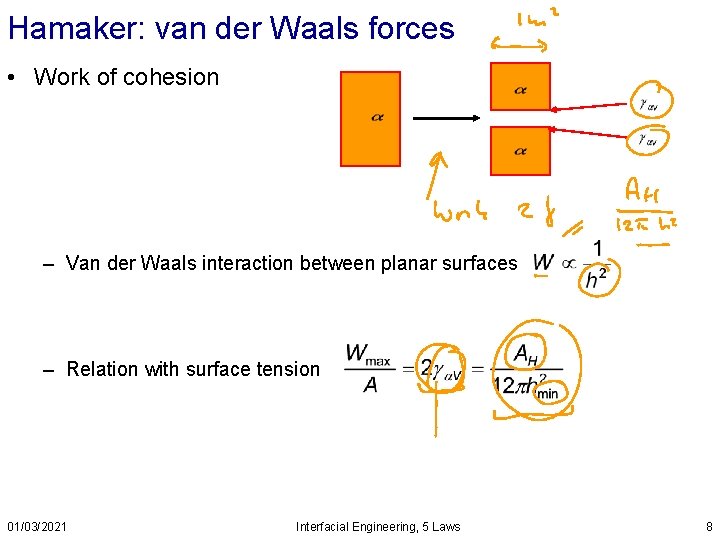
Hamaker: van der Waals forces • Work of cohesion – Van der Waals interaction between planar surfaces – Relation with surface tension 01/03/2021 Interfacial Engineering, 5 Laws 8

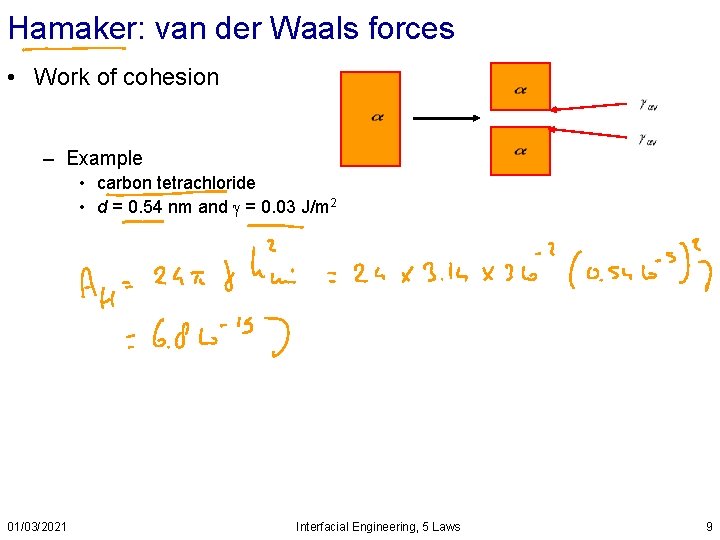
Hamaker: van der Waals forces • Work of cohesion – Example • carbon tetrachloride • d = 0. 54 nm and = 0. 03 J/m 2 01/03/2021 Interfacial Engineering, 5 Laws 9

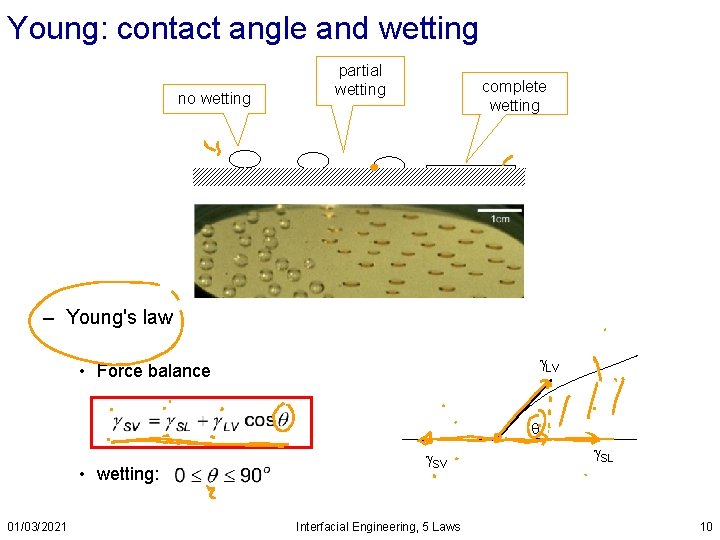
Young: contact angle and wetting no wetting partial wetting complete wetting – Young's law LV • Force balance • wetting: 01/03/2021 SV Interfacial Engineering, 5 Laws SL 10

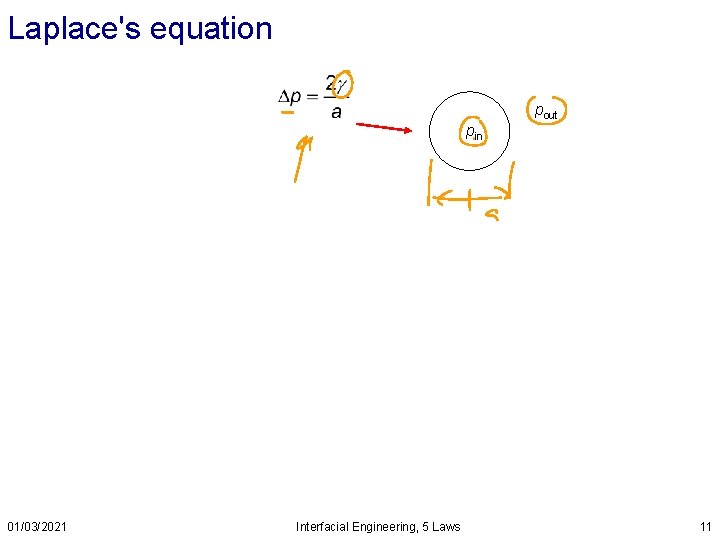
Laplace's equation pout pin 01/03/2021 Interfacial Engineering, 5 Laws 11

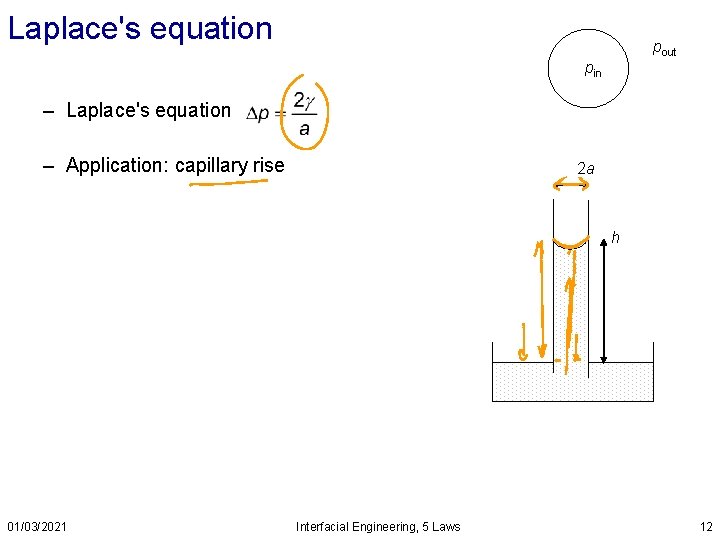
Laplace's equation pout pin – Laplace's equation – Application: capillary rise 2 a h 01/03/2021 Interfacial Engineering, 5 Laws 12

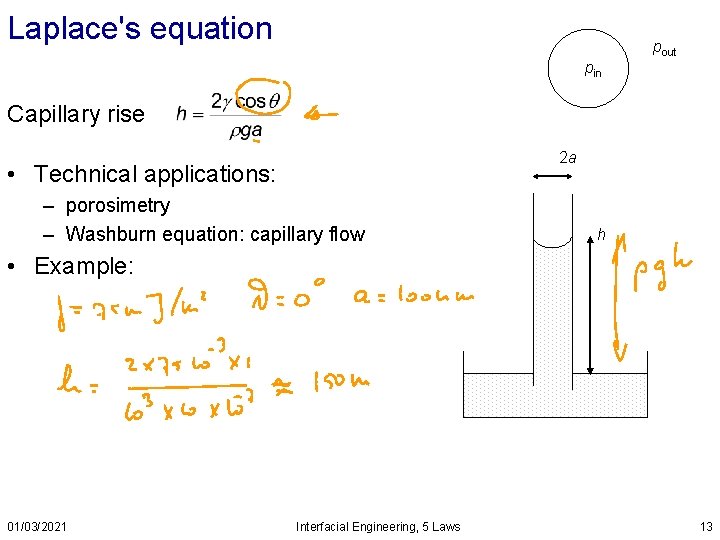
Laplace's equation pout pin Capillary rise 2 a • Technical applications: – porosimetry – Washburn equation: capillary flow h • Example: 01/03/2021 Interfacial Engineering, 5 Laws 13

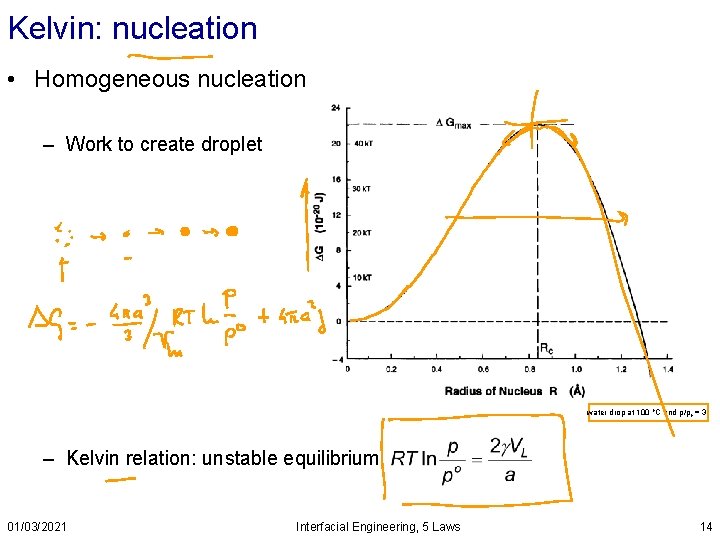
Kelvin: nucleation • Homogeneous nucleation – Work to create droplet water drop at 100 o. C and p/po = 3 – Kelvin relation: unstable equilibrium 01/03/2021 Interfacial Engineering, 5 Laws 14

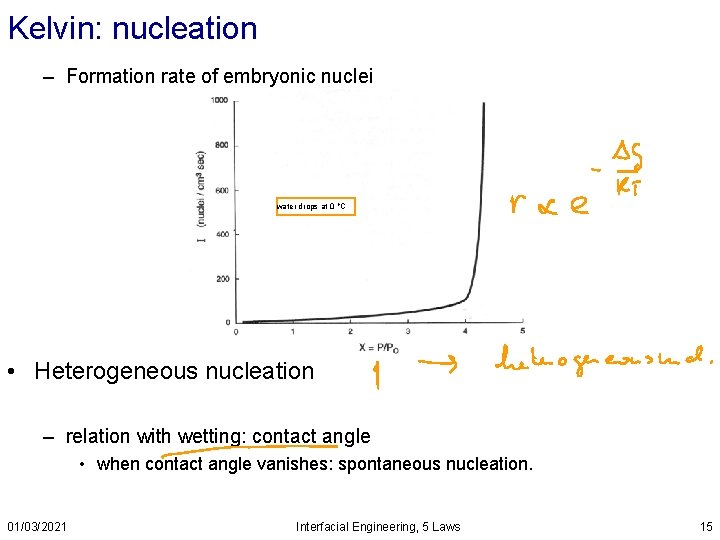
Kelvin: nucleation – Formation rate of embryonic nuclei water drops at 0 o. C • Heterogeneous nucleation – relation with wetting: contact angle • when contact angle vanishes: spontaneous nucleation. 01/03/2021 Interfacial Engineering, 5 Laws 15

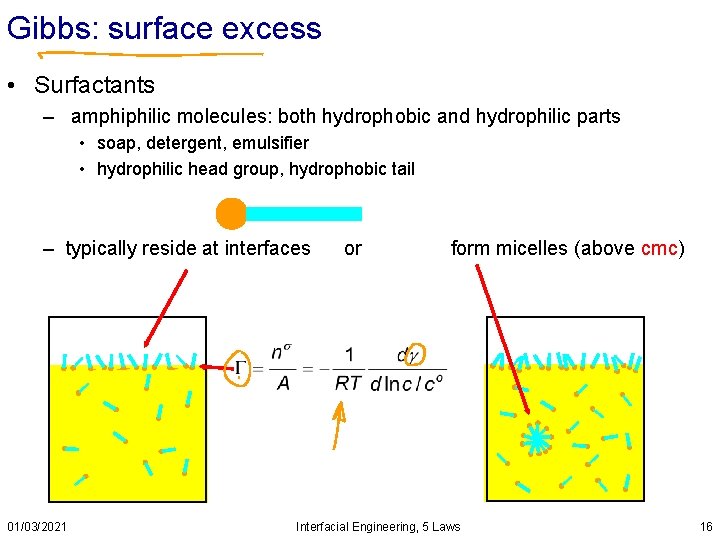
Gibbs: surface excess • Surfactants – amphiphilic molecules: both hydrophobic and hydrophilic parts • soap, detergent, emulsifier • hydrophilic head group, hydrophobic tail – typically reside at interfaces 01/03/2021 or form micelles (above cmc) Interfacial Engineering, 5 Laws 16

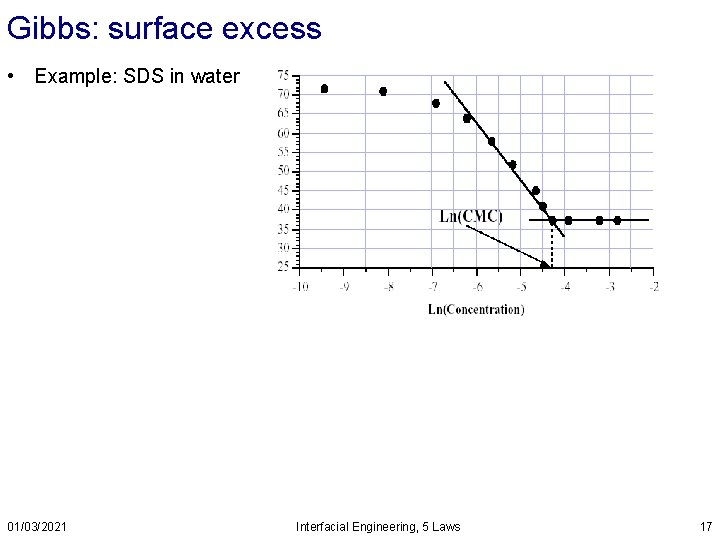
Gibbs: surface excess • Example: SDS in water 01/03/2021 Interfacial Engineering, 5 Laws 17

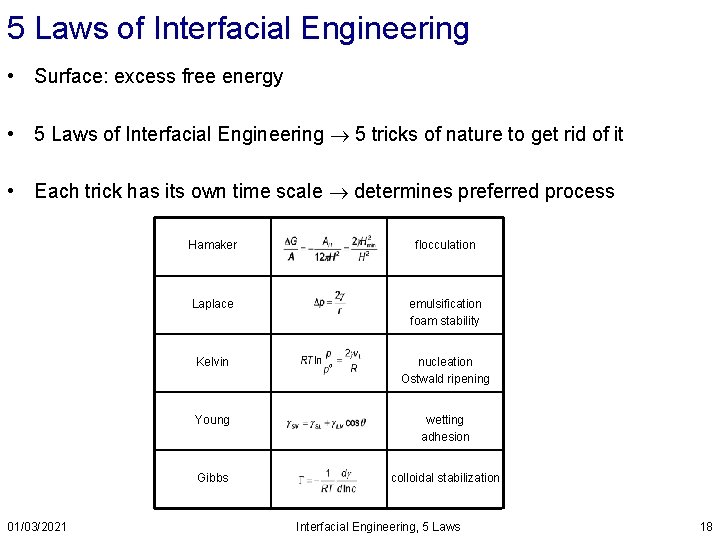
5 Laws of Interfacial Engineering • Surface: excess free energy • 5 Laws of Interfacial Engineering 5 tricks of nature to get rid of it • Each trick has its own time scale determines preferred process 01/03/2021 Hamaker flocculation Laplace emulsification foam stability Kelvin nucleation Ostwald ripening Young wetting adhesion Gibbs colloidal stabilization Interfacial Engineering, 5 Laws 18