CAPE UNIT 1 BIOLOGY BIOMOLECULESCELL AND MOLECULAR BIOLOGY
CAPE UNIT 1 -BIOLOGY BIOMOLECULES-CELL AND MOLECULAR BIOLOGY

Objectives Understand the chemical structure of water, carbohydrates, lipids and proteins and their roles in living organism.

Water and Hydrogen Bonding What A is water? dipolar universal solvent for charged polar molecules and ions which are attracted to the weak charges on the water molecule.

What is water? Water is a chemical substance with the chemical formula H 2 O. Its molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state (water vapor or steam). https: //www. thoughtco. com/definition-of-water-in-chemistry-605946

Definition of key terms Solvent: The substance/s in which the solute dissolves. For example, Water, Gasoline (dissolves Asphalt), Vinegar (dissolves Calcium Carbonate) , Alcohol (dissolves oil) , Kerosene (dissolves iodine). Solute: The substance that dissolves in the solvent

Definition of key terms Solubility: The maximum amount of substance that can be dissolved in fixed amount of solvent to form a stable solution at a given temperature. Insoluble: Compounds that are poorly soluble or do not readily dissolve in a particular solvent; e. g. oil is insoluble in water.

Definition of key terms Cohesion: The sticking together of the electronegative oxygen atom to the hydrogen atoms [O-H] water molecules, due to intramolecular forces by way of covalent bonding.
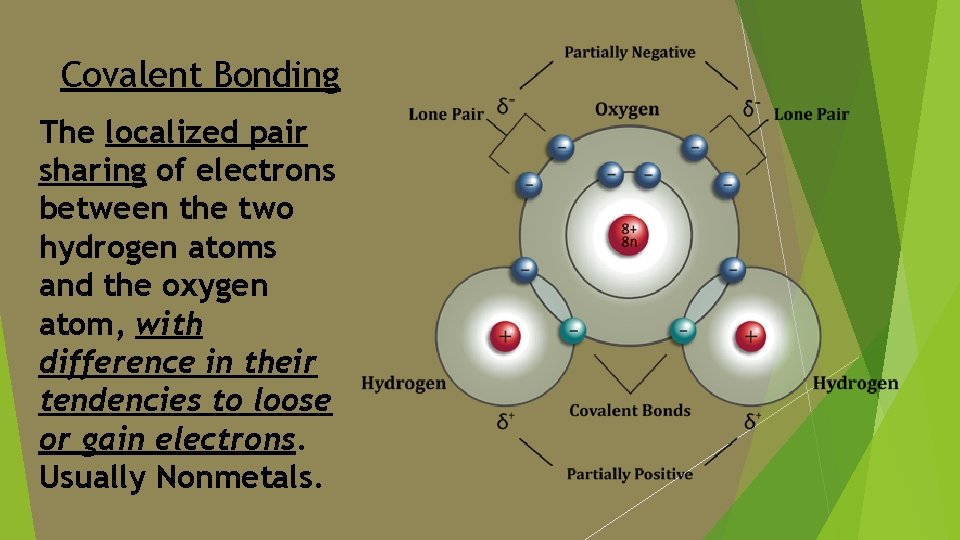
Covalent Bonding The localized pair sharing of electrons between the two hydrogen atoms and the oxygen atom, with difference in their tendencies to loose or gain electrons. Usually Nonmetals.

Definition of key terms is the property of different molecules or surfaces to cling to each other. In water, the molecules [O-H] attach to each other by way of hydrogen bonding due to intermolecular forces (the attractive and repulsive forces among different particles/molecules). Adhesion:
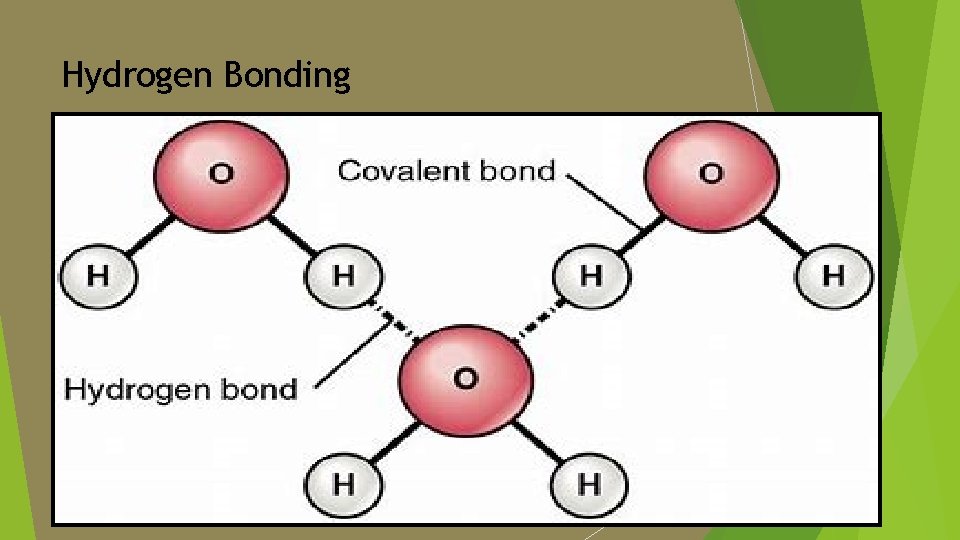
Hydrogen Bonding
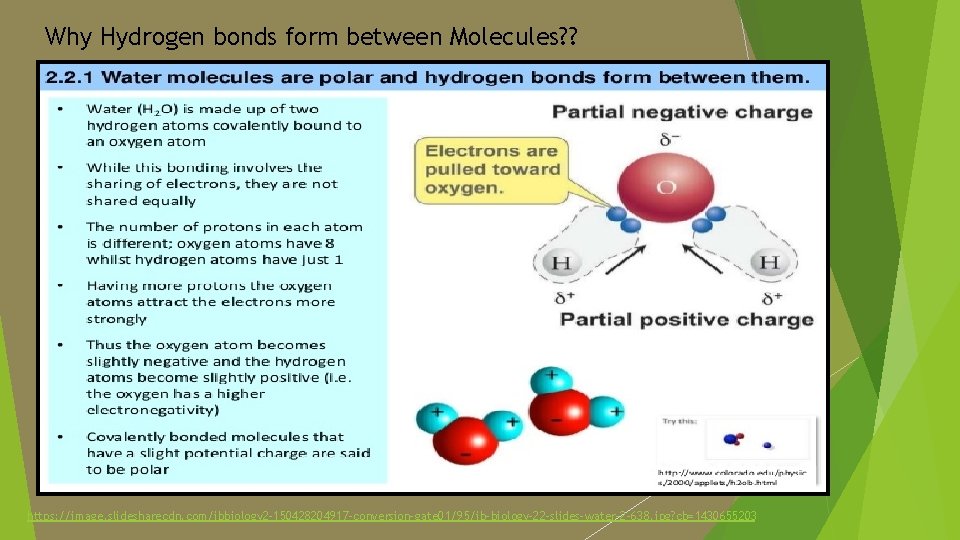
Why Hydrogen bonds form between Molecules? ? https: //image. slidesharecdn. com/ibbiology 2 -150428204917 -conversion-gate 01/95/ib-biology-22 -slides-water-2 -638. jpg? cb=1430655203
List 6 different important properties of water that are due to hydrogen bonding 1. 2. 3. 4. 5. 6.


Why is Water Important to Biology? https: //www. youtube. com/watch? v=JRENt. SROp 3 g

Molecules/Substances that are soluble/insoluble in water Hydrophilic substances: salt, sugar, glucose, soap, vinegar, honey, alcohol, acetone, glycerin. Hydrophobic substances: oil, sand, plastic, silk, rubber, wood, metal, glass, clothe, silk.

The Importance of Water in Cells https: //image. slidesharecdn. com/osmosislessonppt-130410202130 -phpapp 02/95/osmosis-lesson-ppt-5 -638. jpg? cb=1365625325

The Importance of Water in Cells

The Importance of Water in Cells

Which property/ies of Water, accounts for an effective Transport Medium? Justify your answer. 1. 2. 3.

Water as a Transport Medium Water is the medium or substance through which everything is transported in the blood, plasma, lymph, phloem and xylem saps.
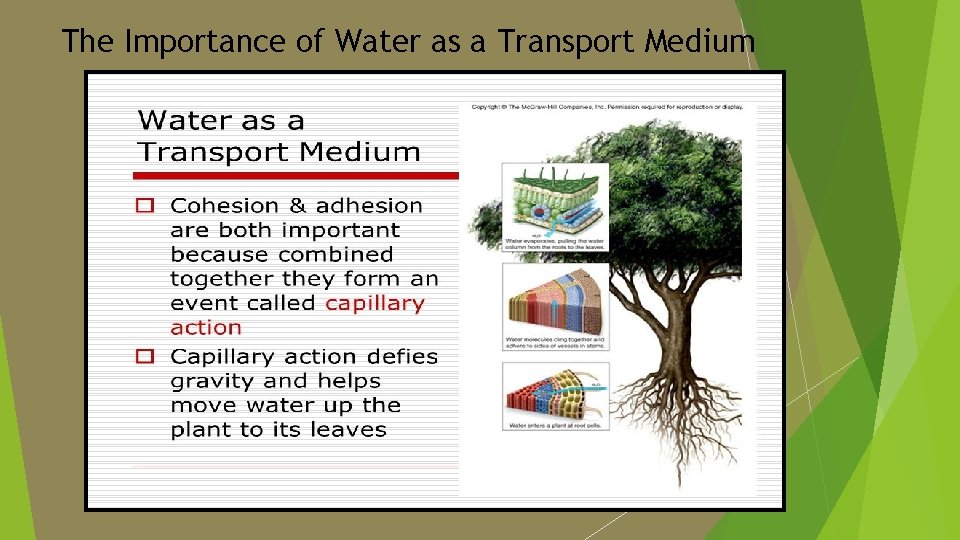
The Importance of Water as a Transport Medium

Which property/ies accounts for water as a Coolant? Justify your answer. 1. 2. 3.

The Importance of Water as a Coolant
The Importance of Water as a Coolant High Specific Heat Capacity: This limits the fluctuations in the temperature of the organisms and the environment of those that live in water. That’s because it takes a lot of thermal energy to break the hydrogen bonds between the water in order to increase the temperature. High latent heat of Vaporization: Loss of water for cooling during transpiration or sweating as a lot of thermal energy is needed to evaporate small quantities of water.

How animals can live indefinitely in cold water without suffering from hypothermia? https: //www. coolantarctica. com/Antarctica%20 fact%20 file/science/c old_all_animals. php

Why does land take less time to heat up during the day than the sea? https: //www. reference. com/science/land-heat-cool -faster-water-f 50 e 45 b 0 f 7 cc 417 b
Discuss the advantages and Disadvantages of water as a habitat. Advantages Coolant Surface tension allows some organisms (mosquitoes) to live on the surface of the water. Aquatic organisms are adapted to breathe in water Disadvantages In very cold water, some warm blooded animals use fur or blubber to regulate their body temperature The hydrogen bonding is not strong enough to support larger organisms. Organisms that do not have the required breathing mechanism may die under water.

What happens to water on Planet Venus? https: //www. youtube. com/watch? v=xcd. Z 1 t. Rz 1 w. U
- Slides: 28