CANCER CHEMOTHERAPY PHARMACOLOGY Leo Mascarenhas MD MS 1





















- Slides: 55

CANCER CHEMOTHERAPY & PHARMACOLOGY Leo Mascarenhas, MD MS 1

Disclosure Information • No financial disclosures • No discussion of unlabeled uses 2

Outline • Syllabus- ABP Outline V. A. 5 (pages 54 -66) • Principles of chemotherapy • Classification/Mechanism of action • Toxicity essentials and drug interactions • Odds and ends 3

Principles of Chemotherapy • Cure – Low therapeutic index • Clinical Trials – Phase 1 - Toxicity and determine MTD – Phase 2 - Response rate and toxicity – Phase 3 - Efficacy vs. standard 4

Principles of Chemotherapy • Multi-drug therapy • Overcome drug resistance to individual agents • Neo-adjuvant vs. adjuvant chemotherapy • Goldie-Coldman hypothesis • Dose intensity • Maximum tolerated dose rate • Supportive care 5

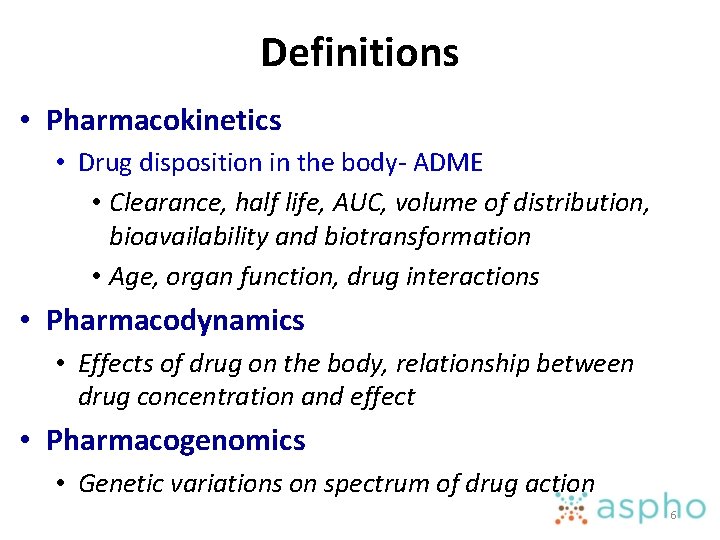
Definitions • Pharmacokinetics • Drug disposition in the body- ADME • Clearance, half life, AUC, volume of distribution, bioavailability and biotransformation • Age, organ function, drug interactions • Pharmacodynamics • Effects of drug on the body, relationship between drug concentration and effect • Pharmacogenomics • Genetic variations on spectrum of drug action 6

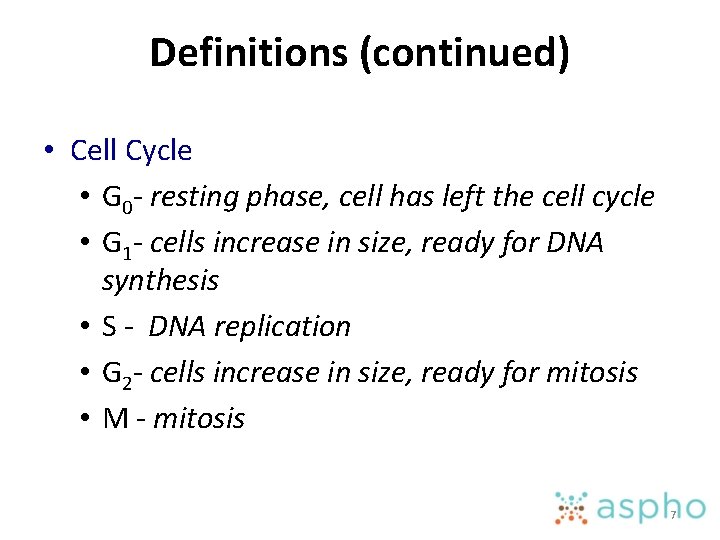
Definitions (continued) • Cell Cycle • G 0 - resting phase, cell has left the cell cycle • G 1 - cells increase in size, ready for DNA synthesis • S - DNA replication • G 2 - cells increase in size, ready for mitosis • M - mitosis 7

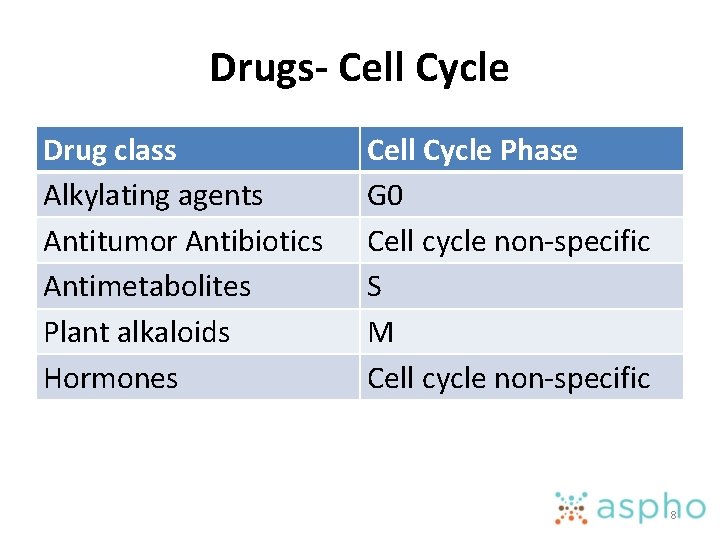
Drugs- Cell Cycle Drug class Alkylating agents Antitumor Antibiotics Antimetabolites Plant alkaloids Hormones Cell Cycle Phase G 0 Cell cycle non-specific S M Cell cycle non-specific 8

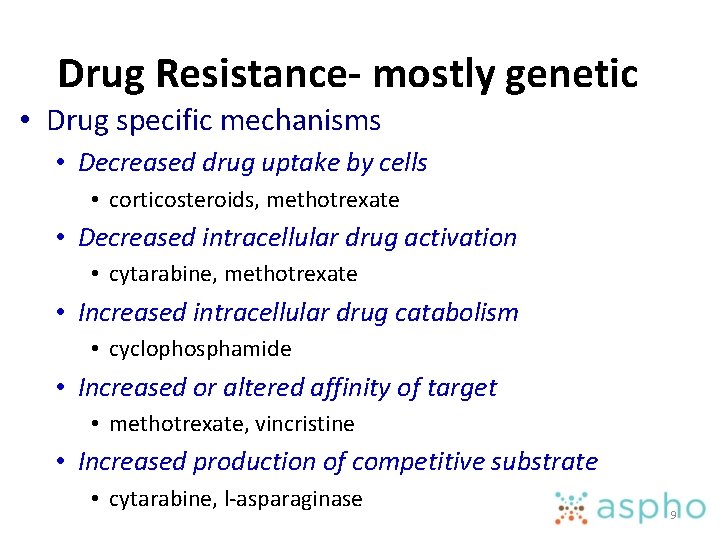
Drug Resistance- mostly genetic • Drug specific mechanisms • Decreased drug uptake by cells • corticosteroids, methotrexate • Decreased intracellular drug activation • cytarabine, methotrexate • Increased intracellular drug catabolism • cyclophosphamide • Increased or altered affinity of target • methotrexate, vincristine • Increased production of competitive substrate • cytarabine, l-asparaginase 9

Drug Resistance (continued) • Multidrug resistance mechanisms • Decreased drug accumulation • Increased P-glycoprotein • Increased drug detoxicfication • Increased Glutathione-S-transferase • Decreased or altered affinity of target • Decreased Topoisomerase II • Increased DNA repair • Increased Alkylguanine-DNA-alkyltransferase • Decreased apoptosis • Increased Bcl-2 expression 10

CNS Pharmacology • Relevance • Primary site- CNS tumors • Sanctuary site- Leukemia • Metastatic site- Solid Tumors • Determinants of CNS penetration • Drug properties • Lipophilicity, molecular size, degree of ionization, free plasma concentration • Cerebral blood flow 11

CNS Pharmacology (continued) • Strategies to enhance CNS penetration • High-dose systemic chemotherapy • Methotrexate, cytarabine • Drugs that penetrate the BBB • nitrosoureas, thiotepa, camptothecins • Disrupting the BBB • mannitol • Administration of drug into the intrathecal space • methotrexate, cytarabine 12

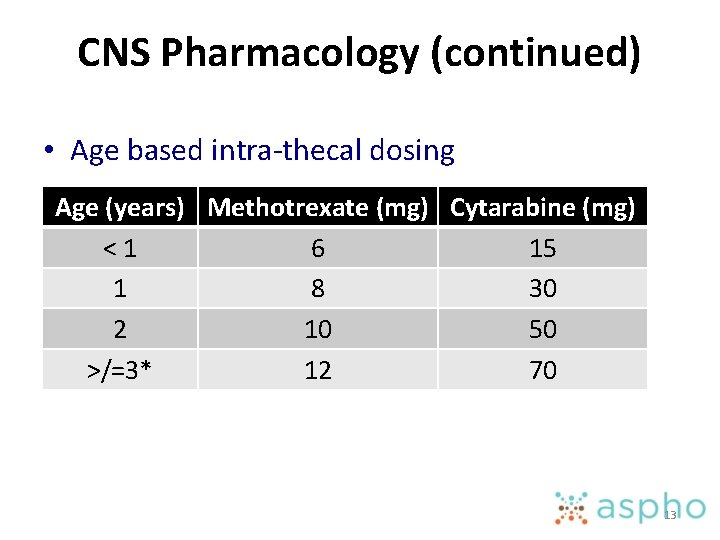
CNS Pharmacology (continued) • Age based intra-thecal dosing Age (years) Methotrexate (mg) Cytarabine (mg) <1 6 15 1 8 30 2 10 50 >/=3* 12 70 13

Classification/Mechanism of Action • • Alkylating Agents Antimetabolites Antibiotics Plant Products Miscellaneous Kinase Inhibitors Other small molecule targeted agents Monoclonal antibodies 14

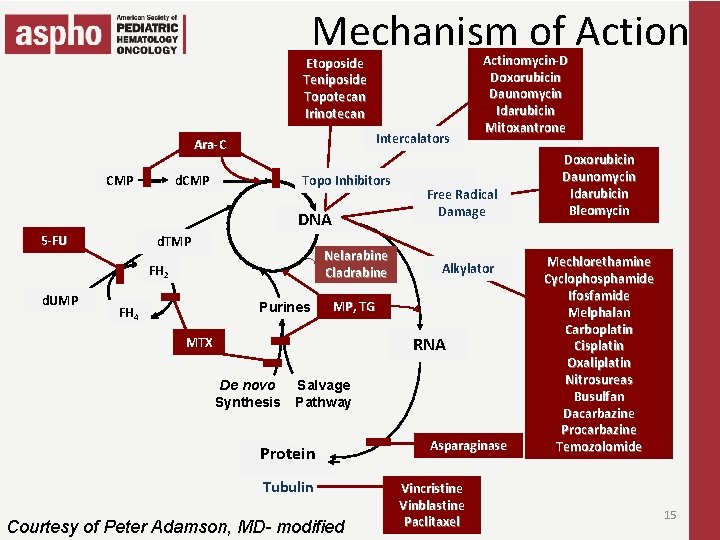
Mechanism of Action Etoposide Teniposide Topotecan Irinotecan Intercalators Ara-C CMP d. CMP Topo Inhibitors DNA 5 -FU d. TMP Nelarabine Cladrabine FH 2 d. UMP Purines FH 4 Actinomycin-D Doxorubicin Daunomycin Idarubicin Mitoxantrone Free Radical Damage Alkylator MP, TG RNA MTX De novo Synthesis Salvage Pathway Protein Tubulin Courtesy of Peter Adamson, MD- modified Asparaginase Vincristine Vinblastine Paclitaxel Doxorubicin Daunomycin Idarubicin Bleomycin Mechlorethamine Cyclophosphamide Ifosfamide Melphalan Carboplatin Cisplatin Oxaliplatin Nitrosureas Busulfan Dacarbazine Procarbazine Temozolomide 15

Alkylating Agents • Nitrogen Mustards • Alkylation, cross-linking • Mechlorethamine, Cyclophosphamide, Ifosfamide, Melphalan • Nitrosoureas • Alkylation, cross-linking, carbamoylation • Lomustine, Carmustine • Platinum compounds • Platination, cross-linking • Cisplatin, Carboplatin, Oxaliplatin 16

Alkylating Agents (continued) • Busulfan • Alkylating, cross-linking • Dacarbazine, Procarbazine, Temozolomide • Methylation, free radical formation • Toxicity– Nausea/vomiting, Myelosuppression, Hemorrhagic cystitis – Infertility, secondary leukemia – Fanconi Syndrome, Neurotoxcity (Ifosfamide) – Pulmonary toxicity (Busulfan) 17

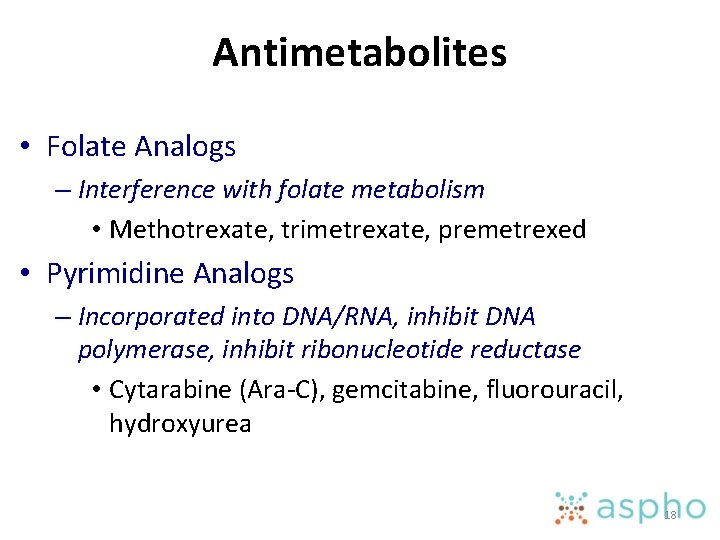
Antimetabolites • Folate Analogs – Interference with folate metabolism • Methotrexate, trimetrexate, premetrexed • Pyrimidine Analogs – Incorporated into DNA/RNA, inhibit DNA polymerase, inhibit ribonucleotide reductase • Cytarabine (Ara-C), gemcitabine, fluorouracil, hydroxyurea 18

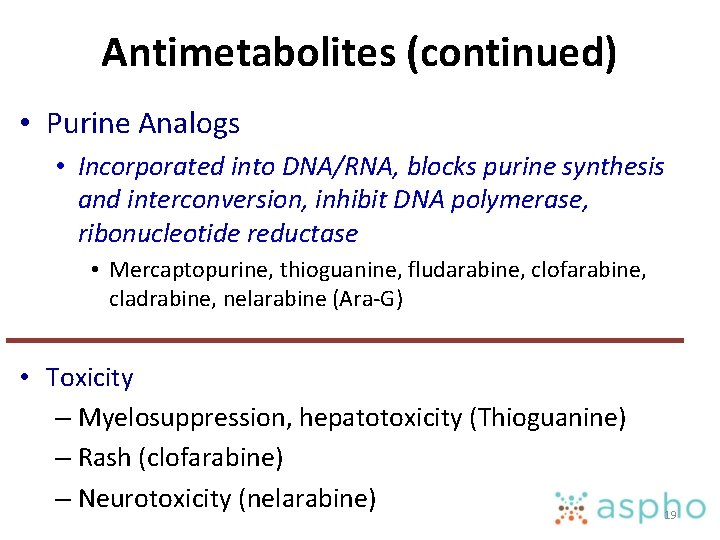
Antimetabolites (continued) • Purine Analogs • Incorporated into DNA/RNA, blocks purine synthesis and interconversion, inhibit DNA polymerase, ribonucleotide reductase • Mercaptopurine, thioguanine, fludarabine, clofarabine, cladrabine, nelarabine (Ara-G) • Toxicity – Myelosuppression, hepatotoxicity (Thioguanine) – Rash (clofarabine) – Neurotoxicity (nelarabine) 19

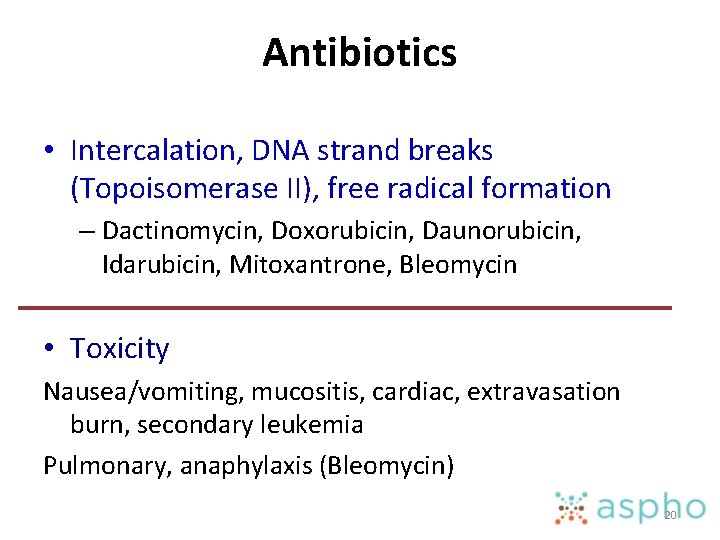
Antibiotics • Intercalation, DNA strand breaks (Topoisomerase II), free radical formation – Dactinomycin, Doxorubicin, Daunorubicin, Idarubicin, Mitoxantrone, Bleomycin • Toxicity Nausea/vomiting, mucositis, cardiac, extravasation burn, secondary leukemia Pulmonary, anaphylaxis (Bleomycin) 20

Plant Products • Vinca Alkaloids – Mitotic inhibitor, blocks microtubule polymerization • Vincristine, vinblastine, vinorelbine • Toxicity Neurotoxicity, SIADH, thrombocytosis (vincristine) Extravasation burn Myelosuppression (Vinblastine, Vinorelbine) 21

Plant Products (continued) • Taxanes – Microtubule inhibitor, blocks microtubule depolymerization • Paclitaxel, docetaxel • Toxicity – Infusion reactions, skin toxicity 22

Miscellaneous (1) • Asparaginase/PEG Asparaginase/Erwinase – Depletes circulating pool of asparagine – Sensitive tumor cells cannot upregulate asparagine synthase – PEGylation decreases immunogenicity and increases half life – Decreases clearance of dexamethasone • Toxicity – Allergy, coagulopathy, hyperglycemia, pancreatitis, hepatotoxicity, encephalopathy 23

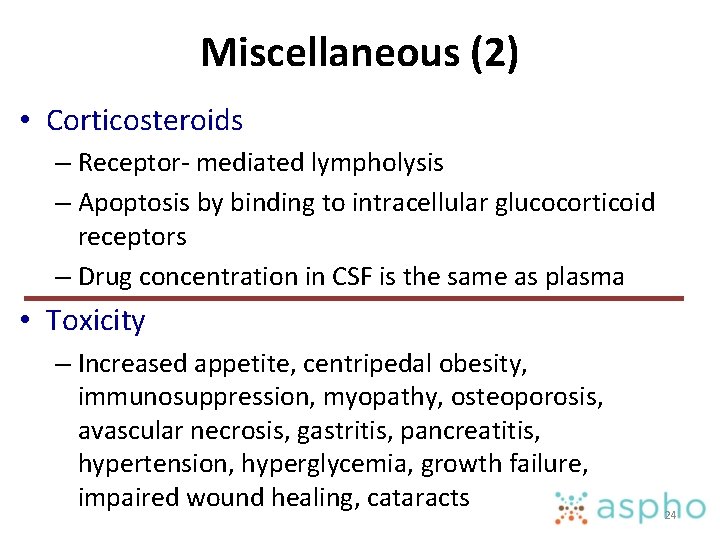
Miscellaneous (2) • Corticosteroids – Receptor- mediated lympholysis – Apoptosis by binding to intracellular glucocorticoid receptors – Drug concentration in CSF is the same as plasma • Toxicity – Increased appetite, centripedal obesity, immunosuppression, myopathy, osteoporosis, avascular necrosis, gastritis, pancreatitis, hypertension, hyperglycemia, growth failure, impaired wound healing, cataracts 24

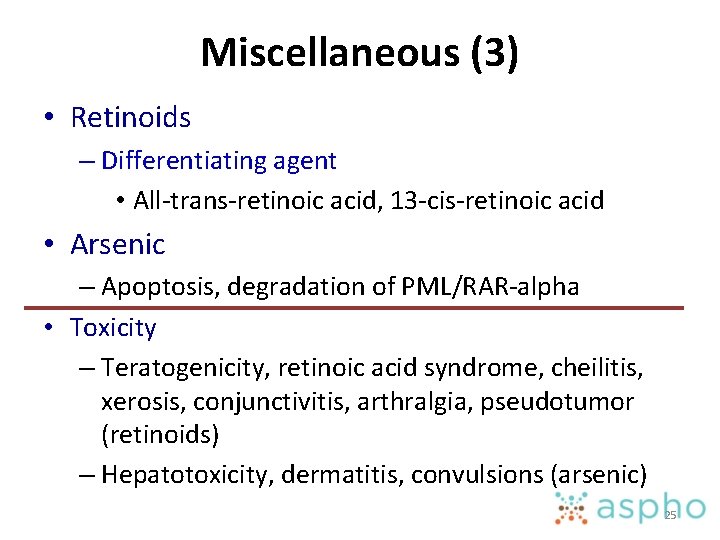
Miscellaneous (3) • Retinoids – Differentiating agent • All-trans-retinoic acid, 13 -cis-retinoic acid • Arsenic – Apoptosis, degradation of PML/RAR-alpha • Toxicity – Teratogenicity, retinoic acid syndrome, cheilitis, xerosis, conjunctivitis, arthralgia, pseudotumor (retinoids) – Hepatotoxicity, dermatitis, convulsions (arsenic) 25

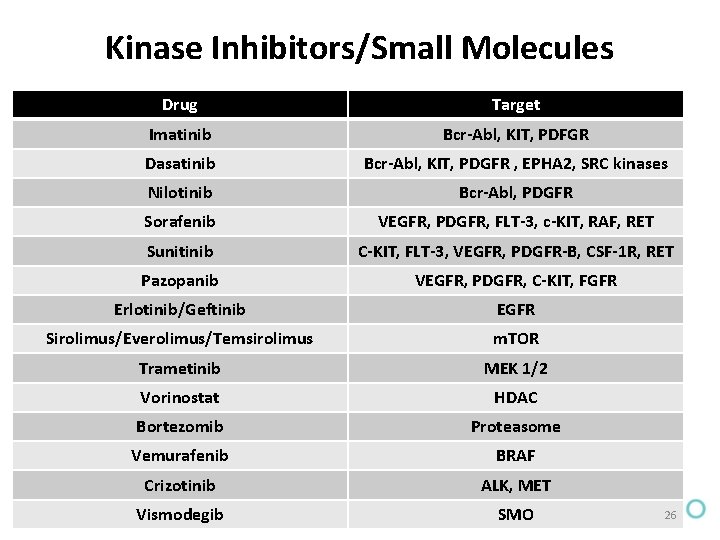
Kinase Inhibitors/Small Molecules Drug Target Imatinib Bcr-Abl, KIT, PDFGR Dasatinib Bcr-Abl, KIT, PDGFR , EPHA 2, SRC kinases Nilotinib Bcr-Abl, PDGFR Sorafenib VEGFR, PDGFR, FLT-3, c-KIT, RAF, RET Sunitinib C-KIT, FLT-3, VEGFR, PDGFR-B, CSF-1 R, RET Pazopanib VEGFR, PDGFR, C-KIT, FGFR Erlotinib/Geftinib EGFR Sirolimus/Everolimus/Temsirolimus m. TOR Trametinib MEK 1/2 Vorinostat HDAC Bortezomib Proteasome Vemurafenib BRAF Crizotinib ALK, MET Vismodegib SMO 26

Monoclonal Antibodies Drug Target Rituximab CD 20 Trastuzumab HER 2 Gemtuzumab ozogamicin CD 33 Alemtuzumab CD 52 90 Y-labeled ibritumomab CD 20 131 Y-labeled tositumomab CD 20 Cetuximab EGFR Bevacizumab VEGF Panitumumab EGFR Ofatumumab CD 20 Ipilumumab CTLA 4 Brentuximab CD 30 Nivolumab/Pembrolizumab PD-1 27

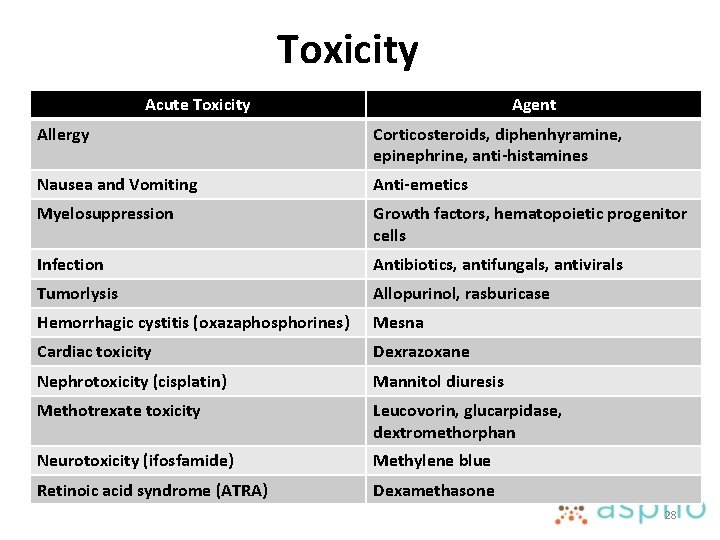
Toxicity Acute Toxicity Agent Allergy Corticosteroids, diphenhyramine, epinephrine, anti-histamines Nausea and Vomiting Anti-emetics Myelosuppression Growth factors, hematopoietic progenitor cells Infection Antibiotics, antifungals, antivirals Tumorlysis Allopurinol, rasburicase Hemorrhagic cystitis (oxazaphosphorines) Mesna Cardiac toxicity Dexrazoxane Nephrotoxicity (cisplatin) Mannitol diuresis Methotrexate toxicity Leucovorin, glucarpidase, dextromethorphan Neurotoxicity (ifosfamide) Methylene blue Retinoic acid syndrome (ATRA) Dexamethasone 28

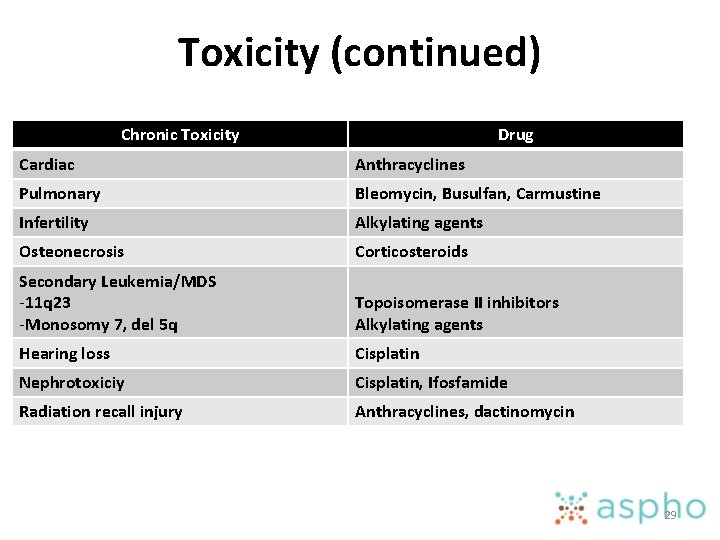
Toxicity (continued) Chronic Toxicity Drug Cardiac Anthracyclines Pulmonary Bleomycin, Busulfan, Carmustine Infertility Alkylating agents Osteonecrosis Corticosteroids Secondary Leukemia/MDS -11 q 23 -Monosomy 7, del 5 q Topoisomerase II inhibitors Alkylating agents Hearing loss Cisplatin Nephrotoxiciy Cisplatin, Ifosfamide Radiation recall injury Anthracyclines, dactinomycin 29

Oxazaphosphorines • Cyclophosphamide (C) and Ifosfamide (I) • Prodrugs • Metabolized to phospho/iphosphoramide mustard and acrolein (hemorrhagic cystitis) • Dechlorethylation I>C (neurotoxicity) • Busulfan blocks activation of cyclophosphamide 30

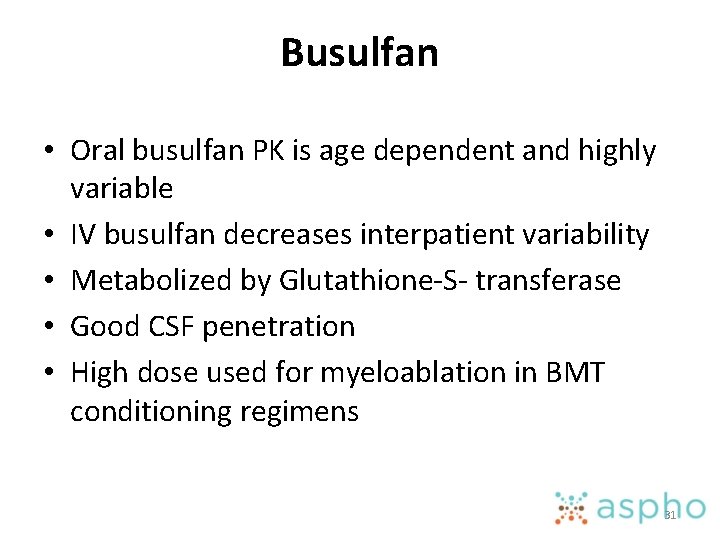
Busulfan • Oral busulfan PK is age dependent and highly variable • IV busulfan decreases interpatient variability • Metabolized by Glutathione-S- transferase • Good CSF penetration • High dose used for myeloablation in BMT conditioning regimens 31

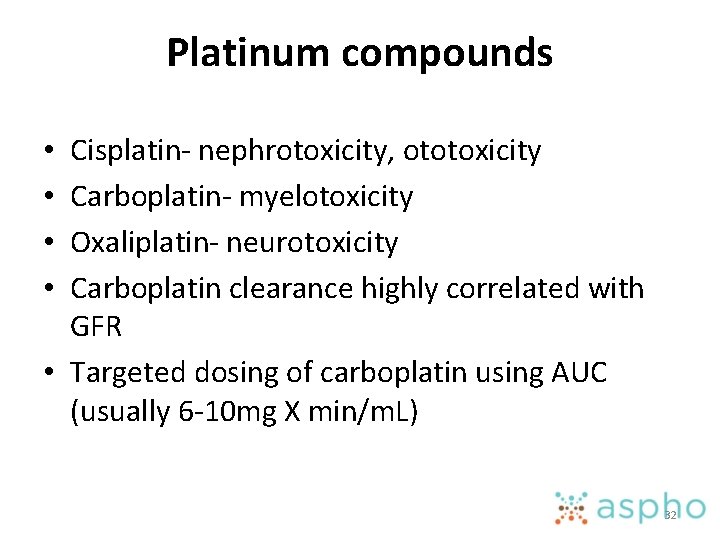
Platinum compounds Cisplatin- nephrotoxicity, ototoxicity Carboplatin- myelotoxicity Oxaliplatin- neurotoxicity Carboplatin clearance highly correlated with GFR • Targeted dosing of carboplatin using AUC (usually 6 -10 mg X min/m. L) • • 32

Thiopurines • MP and TG are prodrugs • MP- TGMP ( 3 steps); TG- TGMP (1 step) • TGMP is phosphorylated to TGTP which is then converted by ribonucleotide reductase to d. TGTP which is incorporated into DNA. • Thiopurine methyl transferase (TPMT)- Smethylation of thiopurines (active metabolites) • Homozygous TPMT deficiency- severe toxicity (510% dosing)- 1 in 300 • Heterozygotes TPMT- more frequent dose reductions 33

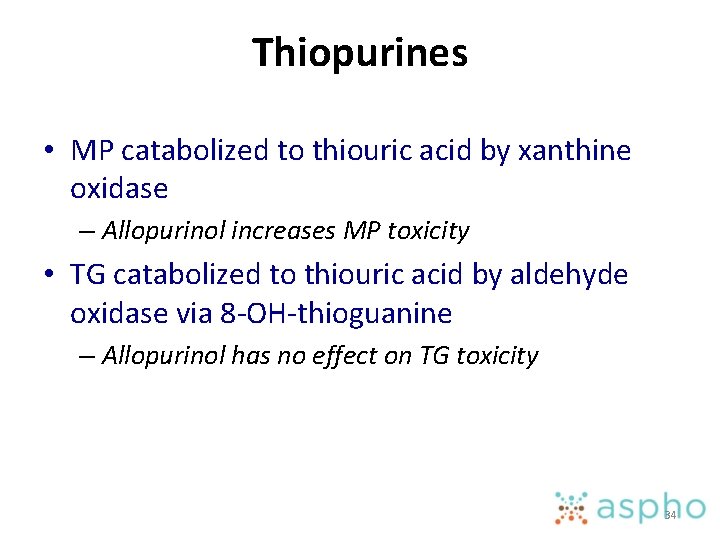
Thiopurines • MP catabolized to thiouric acid by xanthine oxidase – Allopurinol increases MP toxicity • TG catabolized to thiouric acid by aldehyde oxidase via 8 -OH-thioguanine – Allopurinol has no effect on TG toxicity 34

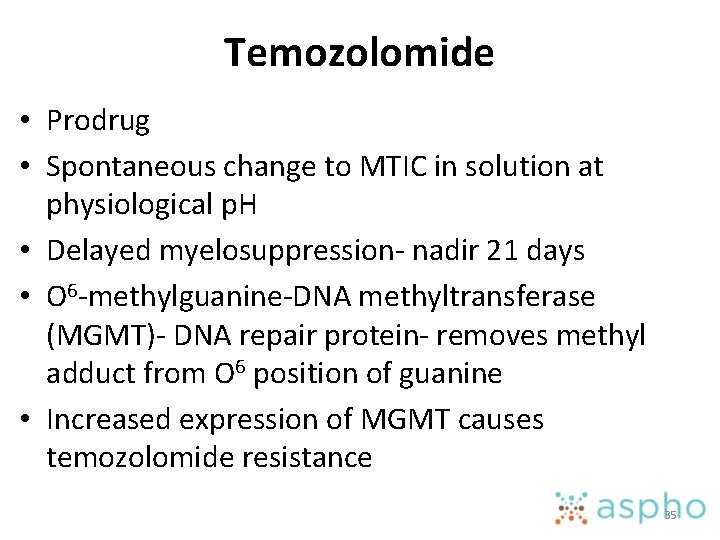
Temozolomide • Prodrug • Spontaneous change to MTIC in solution at physiological p. H • Delayed myelosuppression- nadir 21 days • O 6 -methylguanine-DNA methyltransferase (MGMT)- DNA repair protein- removes methyl adduct from O 6 position of guanine • Increased expression of MGMT causes temozolomide resistance 35

Methotrexate • Inhibits DHFR and prevents conversion of folates to active tetrahydrofolate • Single carbon donor for purine synthesis • Tetrahydrofolate pools depleted • Intracellular methotrexate is polyglutamated and accumulates within the cell leading to apoptosis • Asparaginase depletes glutamine (Capizzi I) rescuing cells from methotrexate toxicity 36

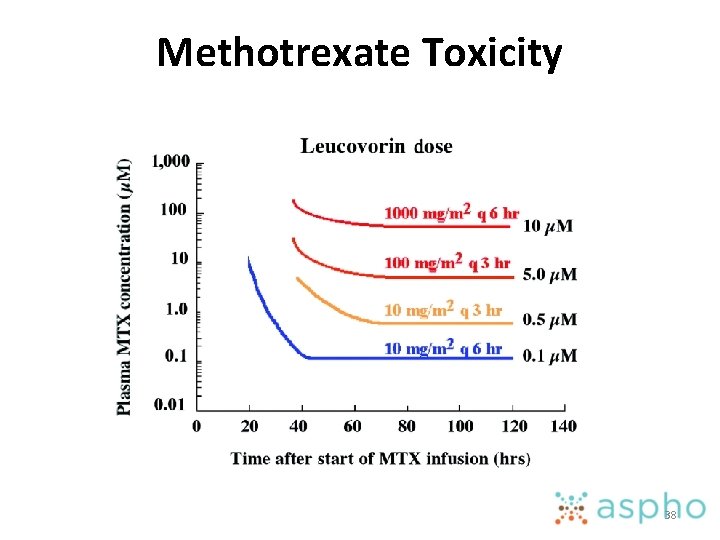
Leucovorin rescue • Does not require DHFR and can be metabolized to tetrahydrofolate • Cancer cells lack transporter for leucovorin • Usually initiated 12 -20 hours after end of methotrexate infusion and continues till serum levels are < 0. 1 micro. M ( some cases 0. 5 micro. M) 37

Methotrexate Toxicity 38

Methotrexate Toxicity • Salicylates, NSAIDS, penicillins, probenicid, sulfasoxazole, iodinated radiographic contrast, proton pump inhibitors • Glucarpidase hydrolyzes methotrexate to DAMPA and glutamic acid • Indicated when serum creatinine X 2 times baseline, 24 hour level > 50 micro. M, 48 hour level > 5 micro. M OR 42 hour level > 10 micro. M and serum creatinine X 1. 5 times baseline • Leucovorin should not be given 2 hours before & after 39

Irinotecan Prodrug- converted in liver and GI tract to SN-38 100 -1000 fold more potent Myelosuppression and diarrhea are dose limiting SN-38 conjugated in liver to SN-38 G by UGT 1 A 1 deficiency (Gilbert, Crigler Najjar) or drugs that inhibit UGT 1 A 1 (valproate, sorafenib) can increase irinotecan toxicity • Bacterial beta-glucoronidase convert SN-38 G to SN 38 causing delayed diarrhea • Cefexime/Cefpodoxime eliminate gut bacteria decreasing the incidence of irinotecan induced diarrhea 40 • • •

Retinoic acid • APML and high risk neuroblastoma • Teratogenic • Retinoic acid syndrome-weight gain, respiratory distress, serous effusions, cardiac and renal toxicity • Prophylaxis with dexamethasone • Both ATRA and cis-RA can cause pseudotumor cerebri 41

Rituximab • First FDA approved monoclonal antibody • Used in the treatment of NHL • Reactivation of hepatitis B infection leading to fulminant hepatitis, liver failure and death. 42

Gemtuzumab Ozogamicin • Monclonal antibody against CD 33 that is expressed on AML cells • Linked to calicheamicin, a cell cytotoxin • Increased risk of veno-occlusive disease of liver independent of BMT • Increased risk of mortality in phase 4 studies. 43

Interferon-a • Human protein (9 p) developed with recombinant DNA technology • Antitumor activity – Hairy cell leukemia, CML, multiple myeloma, follicular lymphoma, renal cell carcinoma, Kaposi sarcoma and melanoma • Bind to specific plasma membrane receptors and stimulates various genes • Antiangiogenic, activation of immune cells and increased immunogenicity of tumor cells, directing gene stimulation 44

Interferon-a (continued) • Toxicity – Chills, rigors, fevers, , malaise, myalgias, mild neutropenia – Fatigue, anorexia, weight loss, transaminitis, depression – Proteinuria, nephrotic syndrome, acute renal failure – Spastic diplegia – Hypothyroidism (antibody mediated) 45

Rasburicase • Recombinant urate oxidase • Converts uric acid to water-soluble allantoin • Anaphylaxis, methhemoglobinemia and hemolysis in patients with G 6 PD deficiency 46

? 47

Mechanisms of Drug Resistance (1) • Alkylating agents – Decreased transport, Increased DNA repair, increased intracellular catabolism, decreased uptake, increased glutathione-S-transferase • Antimetabolites – Decreased transport, increased target enzyme, decreased polyglutamation, increased intracellular catabolism, decreased intracellular activation, increased target enzyme 48

Mechanisms of Drug Resistance (2) • Antibiotics – Multidrug resistance, decreased topoisomerase II, increased intracellular catabolism, increased DNA repair • Vinca alkaloids – Multidrug resistance, altered tubulin subunit • Corticosteroids – Loss or defect in glucocorticoid receptor 49

Mechanisms of Drug Resistance (3) • Asparaginase – increased intracellular asparagine synthase, neutralizing antibodies • Retinoids and Arsenic – Mutations in PML/RAR-alpha • Imatinib – Abl-kinase domain mutations, MDR overexpression 50

Mechanism of Resistance Drug Pharmacologic Defect Decreased uptake Methotrexate expression of folate receptor Decreased activation Ara-C, Fludarabine, Cladribine Methotrexate deoxycytidine kinase folylpolygutamyl synthetase Increased drug target Methotrexate, 5 -FU, Imatinib Amplified DHFR, TS, bcr-abl kinase Altered drug target VP-16, Doxorubicin, Imatinib Altered topo II, DHFR, bcrabl kinase Increased detoxification Alkylators glutathione or glutathione transferase Enhanced DNA repair Alkylators, platinum analogs Nitrosoureas, procarbazine, temozolomide nucleotide excision repair O 6 -alkyl-guanine alkyl transferase Defective recognition of DNA adducts Cisplatin Mismatch repair defect Increased drug efflux Doxorubicin, VP-16, vinca alkaloids, paclitaxel, topotecan MDR expression or MDR gene amplification Defective checkpoint function and apoptosis Most anticancer drugs p 53 mutations 51

ABP Content Outline V. a. 5 (1) Slide # ABP Content 4 5. a 5 5. a, b, c 6 5. a 7 5. a 8 5. a 9 5. c 10 5. c 11 5. a 12 5. a 13 5. a 14 5. a 52

ABP Content Outline V. a. 5 (2) Slide # ABP Content 15 5. a 16 5. d. 1 17 5. d. 1 18 5. d. 2 19 5. d. 2 20 5. d. 3 21 5. d. 5 22 5. d. 5 23 5. d. 17 ab 24 5. d. 5 ab 25 6 27 6 53

ABP Content Outline V. a. 5 (3) Slide # ABP Content 28 6 29 5. d; 7 30 5. d 31 5. d. 12 ab, 13 ab 32 5 d. 20 ab 33 5. d. 18 ab, 19 ab 34 5. d. 1 ab, 2 ab 35 5. d. 1 ab, 2 ab 36 5. d. 23 ab 37 5. d. 3 ab 38 5. d. 3 ab 54

ABP Content Outline V. a. 5 (4) Slide # ABP Content 39 5. d. 3 ab 40 5. d. 3 ab 41 5. d. 22 ab 42 6 43 6 44 6 45 6 46 7 48 5 c 49 5 c 50 5 c 55
 4ac 4t chemotherapy
4ac 4t chemotherapy Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Chemotherapy
Chemotherapy Icd 9 code for oral thrush
Icd 9 code for oral thrush Debra forman
Debra forman Principles of chemotherapy
Principles of chemotherapy Caf education
Caf education General principles of chemotherapy
General principles of chemotherapy First pass effect drugs
First pass effect drugs Rationale meaning in pharmacology
Rationale meaning in pharmacology Chapter 15 diagnostic procedures and pharmacology
Chapter 15 diagnostic procedures and pharmacology If time permits quotes
If time permits quotes First pass metabolism definition pharmacology
First pass metabolism definition pharmacology Fish pharmacology
Fish pharmacology Loading dose formula in pharmacology
Loading dose formula in pharmacology Reverse pharmacology
Reverse pharmacology Basic & clinical pharmacology
Basic & clinical pharmacology What is ion trapping in pharmacology
What is ion trapping in pharmacology Pharmacology pay
Pharmacology pay Clinical pharmacology seminar
Clinical pharmacology seminar Therapeutic index
Therapeutic index Define pharmacology
Define pharmacology Tachyphylaxis in pharmacology
Tachyphylaxis in pharmacology Analglesia
Analglesia Dopamine pharmacology
Dopamine pharmacology Pharmacology chapter 1
Pharmacology chapter 1 Pharmacology for nurses: a pathophysiological approach
Pharmacology for nurses: a pathophysiological approach Venipuncture for radiologic technologists
Venipuncture for radiologic technologists Hepatic extraction ratio formula
Hepatic extraction ratio formula Tactcardia
Tactcardia Npte pharmacology
Npte pharmacology Define pharmacology
Define pharmacology Guaifenasin
Guaifenasin Glomerular
Glomerular Basic & clinical pharmacology
Basic & clinical pharmacology Metabolism definition in pharmacology
Metabolism definition in pharmacology Ansc 497
Ansc 497 Focus on pharmacology essentials for health professionals
Focus on pharmacology essentials for health professionals What is ion trapping in pharmacology
What is ion trapping in pharmacology Efficacy definition pharmacology
Efficacy definition pharmacology What are the different routes of drug administration
What are the different routes of drug administration Define pharmacology
Define pharmacology Pharmacology tutor anderson
Pharmacology tutor anderson Respiratory pharmacology quiz
Respiratory pharmacology quiz Potentiation example
Potentiation example Clinical pharmacology powered by clinicalkey
Clinical pharmacology powered by clinicalkey Clinical pharmacology seminar
Clinical pharmacology seminar What is pharmacology
What is pharmacology Formula of maintenance dose
Formula of maintenance dose Loading dose
Loading dose Objectives of pharmacology
Objectives of pharmacology Clinical pharmacology residency
Clinical pharmacology residency Chapter 30 principles of pharmacology
Chapter 30 principles of pharmacology Toxicology and applied pharmacology
Toxicology and applied pharmacology Lomotib
Lomotib Basic principles of pharmacology
Basic principles of pharmacology