CAN WE PREVENT NECROTIZING ENTEROCOLITIS NEC Reese H


































- Slides: 66

CAN WE PREVENT NECROTIZING ENTEROCOLITIS (NEC)? Reese H Clark, MD Vice-President and Co-Director The Center for Research, Education, and Quality Pediatrix Medical Group

OBJECTIVES • To define the term NEC • Review the evidence that NEC can be reduced. • Discuss implementation of specific approaches to reduce NEC

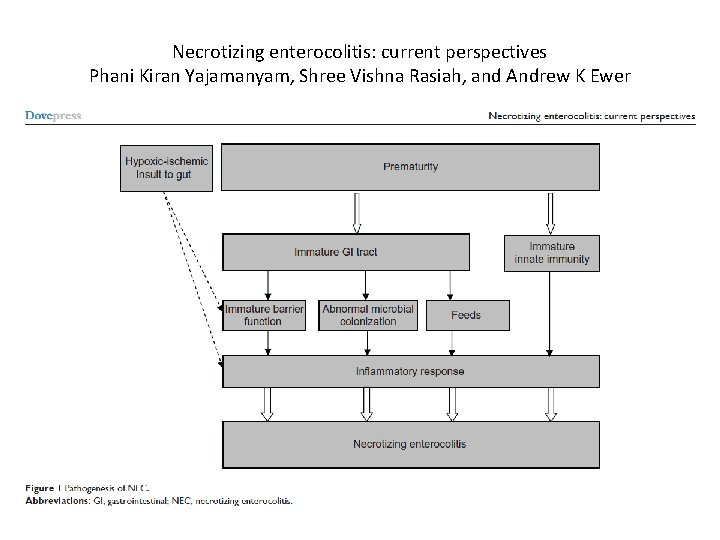
WHAT IS NEC? • It is a window of developmental vulnerability in which innate immunity can be over-activated and the resulting inflammation triggers necrotic obliteration of the neonatal intestinal mucosa • It occurs at a stereotypic time in relation to the gestation at birth (the younger the gestation the later the onset) • It requires that the infant be fed (substantially) • It almost never occurs when the intestinal immune system is mature and intact, even if other risk factors are present • The triggering of NEC is a multi-factorial phenomena, much like BPD, many risk factors heighten neonatal intestinal inflammation until an irreversible “waterline” is surpassed and mucosal necrosis proceeds

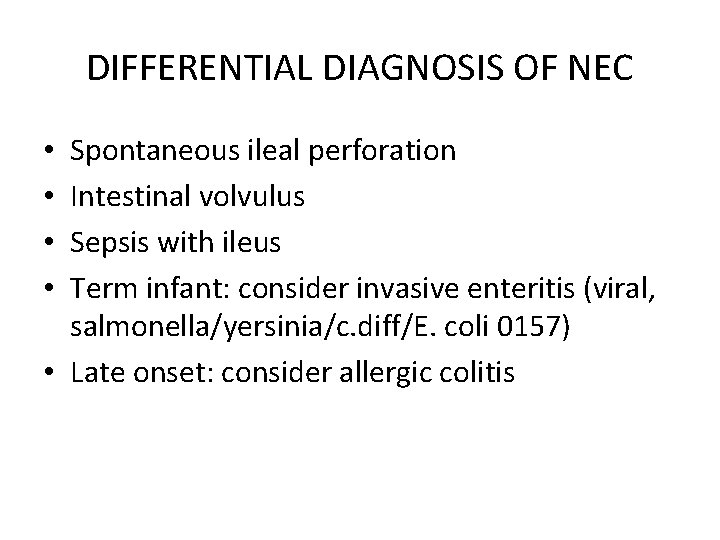
DIFFERENTIAL DIAGNOSIS OF NEC Spontaneous ileal perforation Intestinal volvulus Sepsis with ileus Term infant: consider invasive enteritis (viral, salmonella/yersinia/c. diff/E. coli 0157) • Late onset: consider allergic colitis • •

PROPOSED DEFINITION FOR NEC PHILL GORDON • Acute abdominal distention from focal or global ileus while on > 40 ml/kg/day of enteral feeds – AND • • Radiographic pneumatosis on day of diagnosis OR persistent platelet consumption > 4 days from day of diagnosis OR portal air on the day of diagnosis (not to include iatrogenic) OR surgical / autopsy confirmation of > 4 cm of necrotic bowel with pneumatosis that extends beyond the immediate margins of a solitary perforation. Focal gangrene that principally affects the serosa surrounding a solitary perforation should not be labelled “NEC”. • Things that should NOT be used as diagnostic criteria for NEC – Pneumoperitoneum – confounded by SIP / congenital sources of perforation – Bloody stools – confounded by cows milk intolerance / colitis / iron supplements

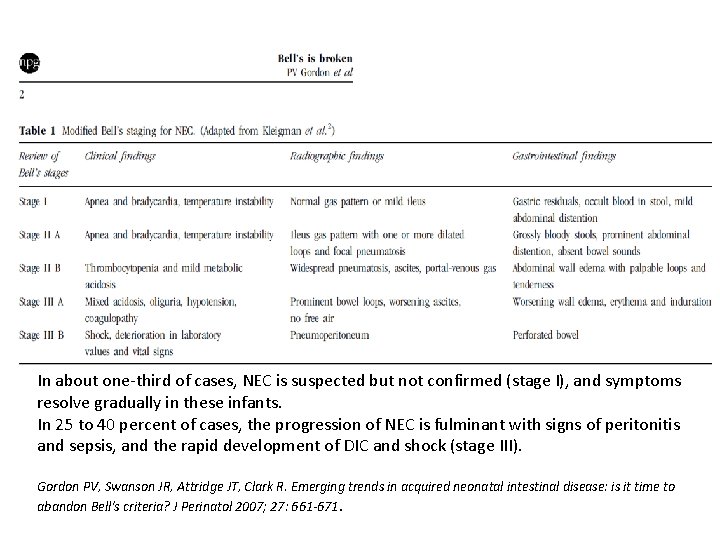
In about one-third of cases, NEC is suspected but not confirmed (stage I), and symptoms resolve gradually in these infants. In 25 to 40 percent of cases, the progression of NEC is fulminant with signs of peritonitis and sepsis, and the rapid development of DIC and shock (stage III). Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell's criteria? J Perinatol 2007; 27: 661 -671.

Spontaneous Intestinal Perforations • Is unrelated to feeds • Is always a surgical disease (and thus is a common surgical NEC contaminant) • Occurs at an earlier time of life than NEC • Affects a younger gestational population • Has unique risk factors (early postnatal steroids and NSAIDs) which are not associated with NEC • Has different histology/pathology from NEC

SPONTANEOUS INTESTINAL PERFORATION (SIP) • Typically found at the terminal ileum. • Occurs primarily in premature, very low birth weight (VLBW, birth weight <1000 g) infants • SIP generally presents within the first 10 days of life as an acute onset of abdominal distension and hypotension. • The classical physical finding is a black-bluish discoloration of the abdominal wall. Abdominal wall erythema, crepitus, and induration commonly seen with NEC rarely present in infants with SIP. • Long-term survival of infants with SIP has improved over the past 30 years with reported survival rates of 64 to 90 percent. Survivors are at risk for neurodevelopmental impairment.

Timing



SYMPTOMS (MAY COME ON SLOWLY OR SUDDENLY) • • Abdominal distention Apnea and bradycardia Blood in the stool Diarrhea Feeding intolerance Lethargy Temperature instability Vomiting

PROPOSED DEFINITION FOR NEC PHIL GORDON • Acute abdominal distention from focal or global ileus while on > 40 ml/kg/day of enteral feeds – AND • • Radiographic pneumatosis on day of diagnosis OR persistent platelet consumption > 4 days from day of diagnosis OR portal air on the day of diagnosis (not to include iatrogenic) OR surgical / autopsy confirmation of > 4 cm of necrotic bowel with pneumatosis that extends beyond the immediate margins of a solitary perforation. Focal gangrene that principally affects the serosa surrounding a solitary perforation should not be labelled “NEC”. • Things that should NOT be used as diagnostic criteria for NEC – Pneumoperitoneum – confounded by SIP / congenital sources of perforation – Bloody stools – confounded by cows milk intolerance / colitis / iron supplements

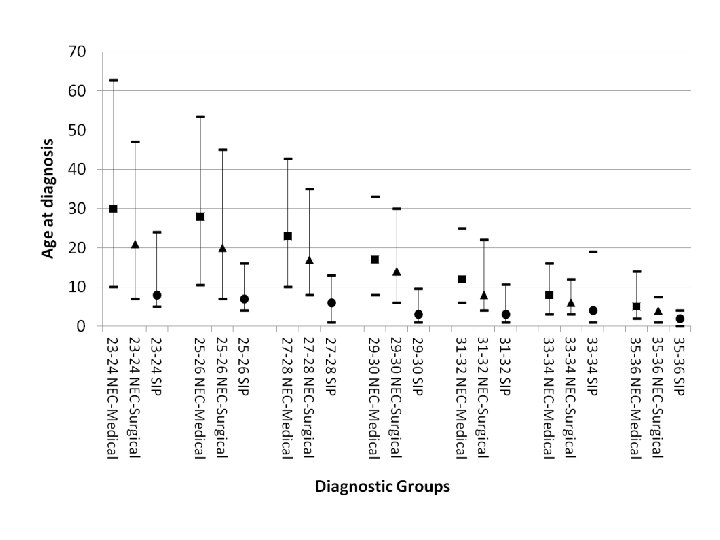
Who gets NEC?

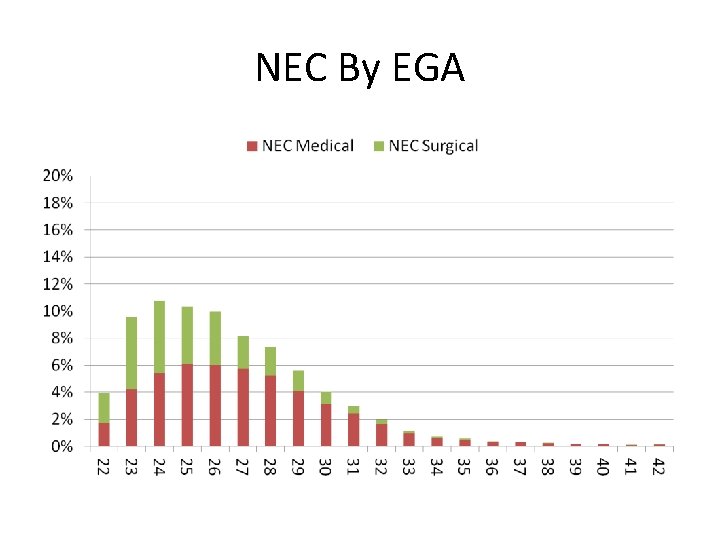
NEC By EGA
WHAT KILLS INFANTS WITH NEC?

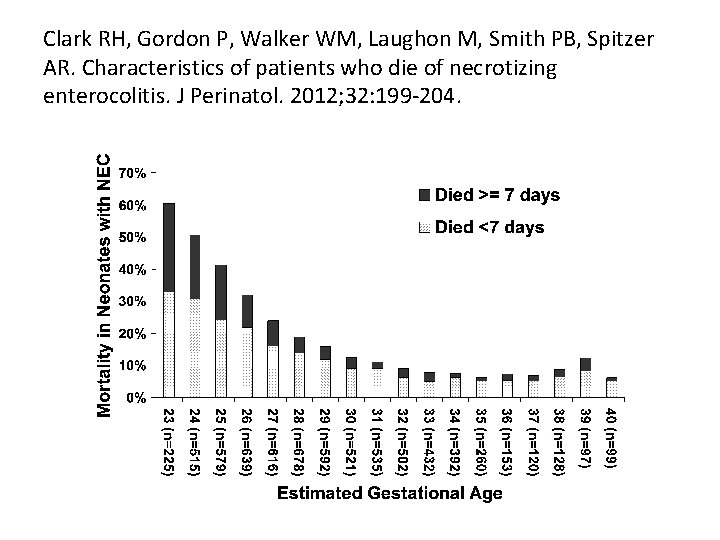
RISK FACTORS ASSOCIATED WITH DEATH FROM NEC • Lower estimated gestational age • Lower birth weight • Treatment with assisted ventilation on the day of diagnosis of NEC • Treatment with vasopressors at the time of diagnosis • Black race • Patients who received only ampicillin and gentamicin on the day of diagnosis were less likely to die.

RISK FACTORS ASSOCIATED WITH RAPID DEATH FROM NEC • Two-thirds of NEC deaths occurred quickly (<7 days from diagnosis), with a median time of death of one day from time of diagnosis. • Infants who died within 7 days of diagnosis had a higher birth weight, more often were on vasopressors and high frequency ventilation at the time of diagnosis compared with patients who died at 7 or more days.

Clark RH, Gordon P, Walker WM, Laughon M, Smith PB, Spitzer AR. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. 2012; 32: 199 -204.

PREVENTING NEC

Necrotizing enterocolitis: current perspectives Phani Kiran Yajamanyam, Shree Vishna Rasiah, and Andrew K Ewer

IMPORTANT INTERVENTIONS THAT REDUCE THE RISK OF NEC • Human milk (both mother’s & donor milk programs) • Standardized feeding guidelines (including early initiation) • Probiotics • Antibiotic stewardship • Optimization of enteral nutrition and growth • Elimination of H 2 blockers and acid pump suppressors • Elimination of cows milk products • Transfusion protocols and transfusion outcome monitoring

HUMAN MILK

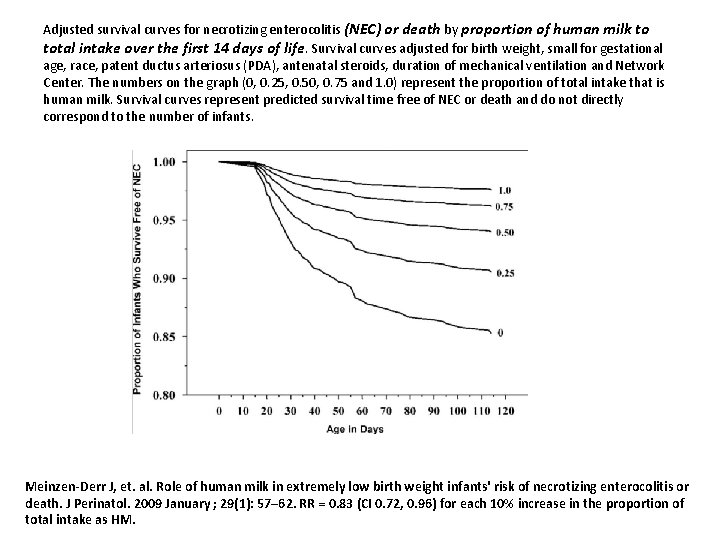
Adjusted survival curves for necrotizing enterocolitis (NEC) or death by proportion of human milk to total intake over the first 14 days of life. Survival curves adjusted for birth weight, small for gestational age, race, patent ductus arteriosus (PDA), antenatal steroids, duration of mechanical ventilation and Network Center. The numbers on the graph (0, 0. 25, 0. 50, 0. 75 and 1. 0) represent the proportion of total intake that is human milk. Survival curves represent predicted survival time free of NEC or death and do not directly correspond to the number of infants. Meinzen-Derr J, et. al. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009 January ; 29(1): 57– 62. RR = 0. 83 (CI 0. 72, 0. 96) for each 10% increase in the proportion of total intake as HM.

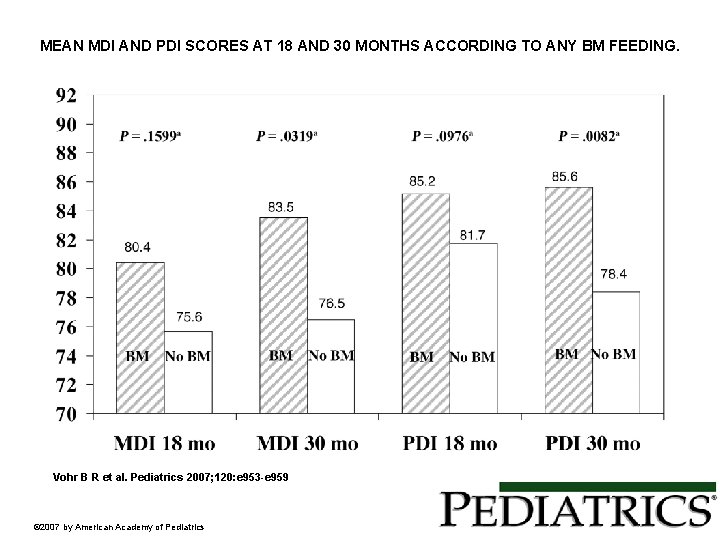
MEAN MDI AND PDI SCORES AT 18 AND 30 MONTHS ACCORDING TO ANY BM FEEDING. Vohr B R et al. Pediatrics 2007; 120: e 953 -e 959 © 2007 by American Academy of Pediatrics

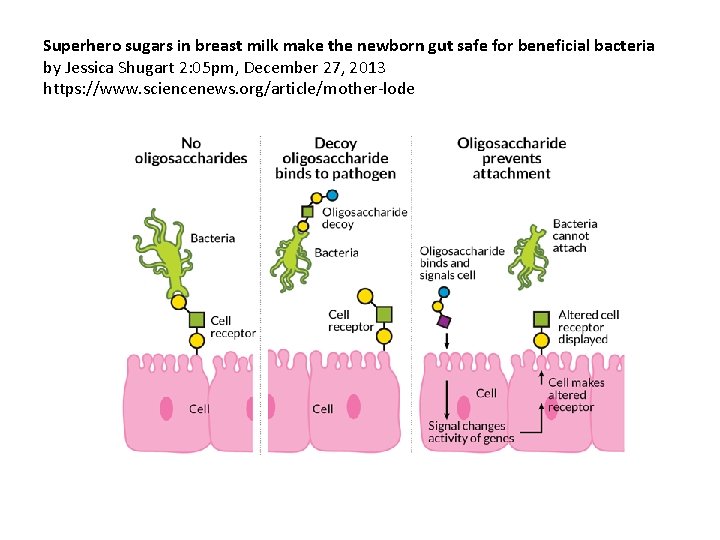
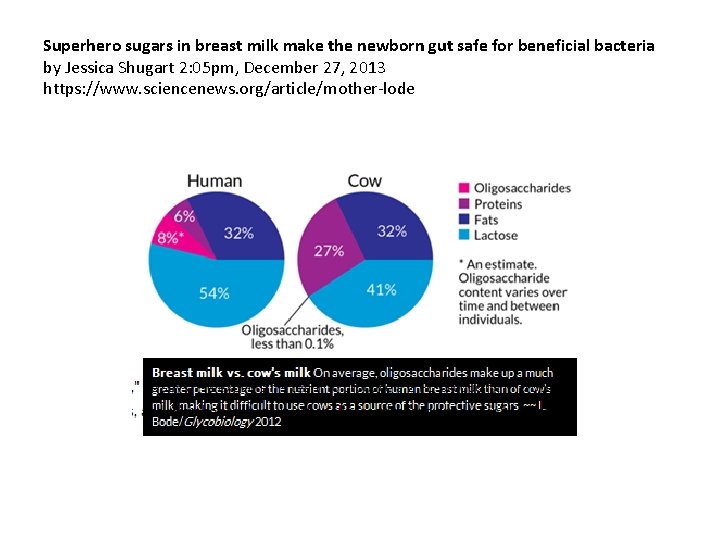
Superhero sugars in breast milk make the newborn gut safe for beneficial bacteria by Jessica Shugart 2: 05 pm, December 27, 2013 https: //www. sciencenews. org/article/mother-lode

Superhero sugars in breast milk make the newborn gut safe for beneficial bacteria by Jessica Shugart 2: 05 pm, December 27, 2013 https: //www. sciencenews. org/article/mother-lode

COLOSTRUM FEEDINGS • Colostrum is a pre-milk fluid rich in immunoglobulins and immune cells that is produced during the first 24 -48 hours postpartum. • It is very like a medicine and may play an important role in protecting preterm infants

HUMAN MILK - DONOR

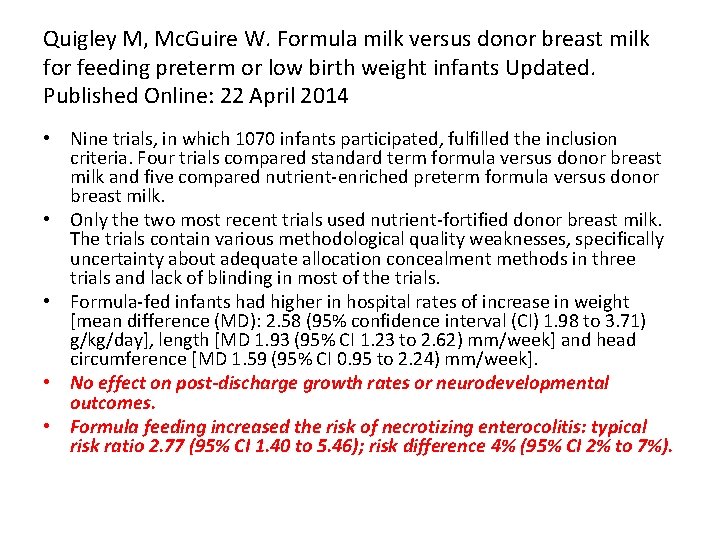
Quigley M, Mc. Guire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants Updated. Published Online: 22 April 2014 • Nine trials, in which 1070 infants participated, fulfilled the inclusion criteria. Four trials compared standard term formula versus donor breast milk and five compared nutrient-enriched preterm formula versus donor breast milk. • Only the two most recent trials used nutrient-fortified donor breast milk. The trials contain various methodological quality weaknesses, specifically uncertainty about adequate allocation concealment methods in three trials and lack of blinding in most of the trials. • Formula-fed infants had higher in hospital rates of increase in weight [mean difference (MD): 2. 58 (95% confidence interval (CI) 1. 98 to 3. 71) g/kg/day], length [MD 1. 93 (95% CI 1. 23 to 2. 62) mm/week] and head circumference [MD 1. 59 (95% CI 0. 95 to 2. 24) mm/week]. • No effect on post-discharge growth rates or neurodevelopmental outcomes. • Formula feeding increased the risk of necrotizing enterocolitis: typical risk ratio 2. 77 (95% CI 1. 40 to 5. 46); risk difference 4% (95% CI 2% to 7%).

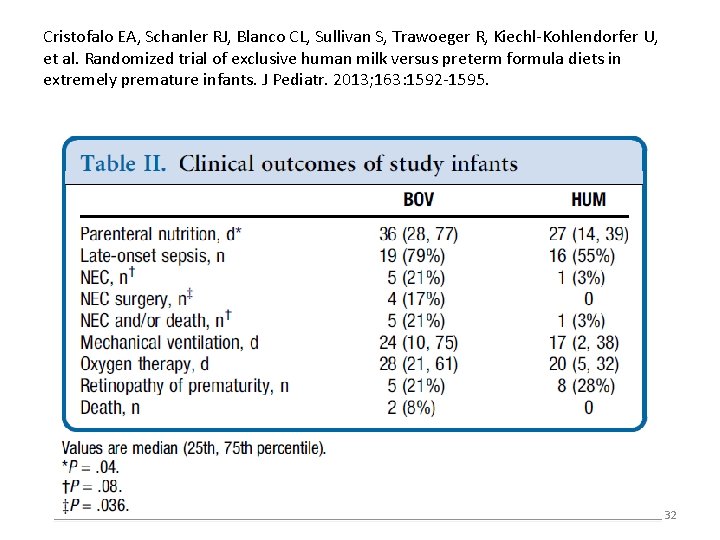
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl. Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013; 163: 1592 -1595. • A multicenter randomized trial in extremely preterm infants compared a feeding regimen based exclusively on human milk (donor breast milk with fortifier derived from human milk) to one using preterm formula and fortifier derived from cow’s milk • Infants were eligible if their mothers did not provide their own breast milk • Infants fed with the human milk regimen had a shorter duration of parenteral nutrition and a lower rate of surgical NEC, but the rate of NEC overall was not statistically different in the two groups. • The incidence of NEC in the group fed formula was very high (21%), much higher than the usual rate of NEC 31

Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013; 163: 1592 -1595. 32

What about human milk and growth?

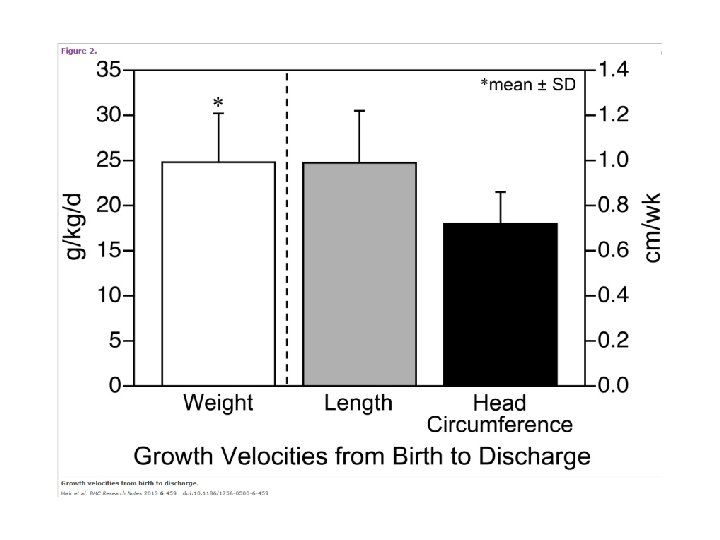
Hair AB, Hawthorne KM, Chetta KE, Abrams SA. Human milk feeding supports adequate growth in infants </= 1250 grams birth weight. BMC Res Notes. 2013; 6: 459. • Single center, prospective observational cohort study, preterm infants weighing ≤ 1250 g BW were fed an exclusive human milk-based diet until 34 wks postmenstrual age • Evaluated 104 infants with mean EGA of 27. 6 ± 2. 0 wks and BW of 913 ± 181 gm • Human milk fortification with donor human milk derived fortifier was started at 60 m. L/kg/d and advanced to provide 6 to 8 additional kilocalories per ounce • Weight gain was 24. 8 ± 5. 4 g/kg/day with length 0. 99 ± 0. 23 cm/week and head circumference 0. 72 ± 0. 14 cm/week. • Weight velocity was affected by day of fortification and day of full feeds


Feeding

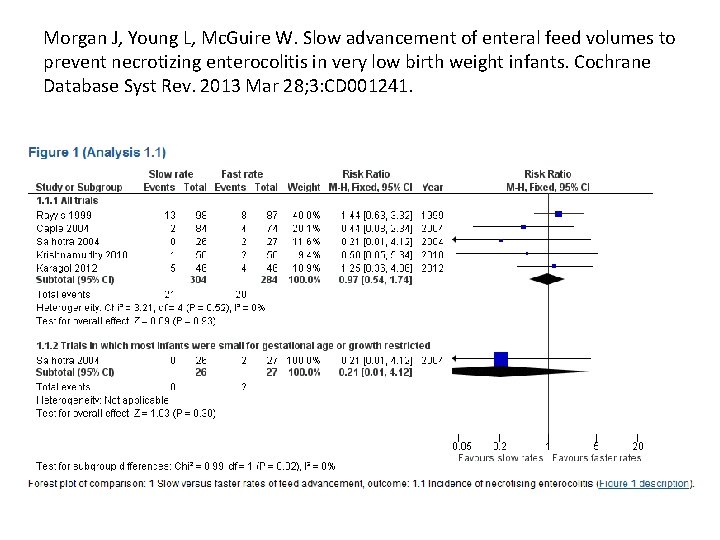
Morgan J, Young L, Mc. Guire W. Slow advancement of enteral feed volumes to prevent necrotizing enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2013 Mar 28; 3: CD 001241. • Five randomized controlled trials in which a total of 588 infants participated. • Few participants were extremely preterm, extremely low birth weight or growth restricted. • The trials defined slow advancement as daily increments of 15 to 20 ml/kg and faster advancement as 30 to 35 ml/kg. • Meta-analyses did not detect statistically significant effects on the risk of necrotizing enterocolitis (typical risk ratio (RR) 0. 97, 95% confidence interval (CI) 0. 54 to 1. 74) or all-cause mortality (RR 1. 41, 95% CI 0. 81 to 2. 74). • Infants who had slow advancement took significantly longer to regain birth weight (reported median differences two to six days) and to establish full enteral feeding (two to five days).

Morgan J, Young L, Mc. Guire W. Slow advancement of enteral feed volumes to prevent necrotizing enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2013 Mar 28; 3: CD 001241.

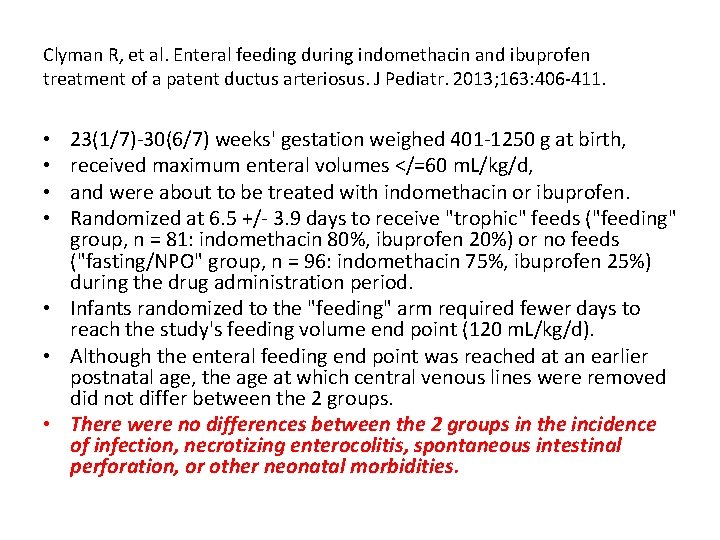
Clyman R, et al. Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus. J Pediatr. 2013; 163: 406 -411. 23(1/7)-30(6/7) weeks' gestation weighed 401 -1250 g at birth, received maximum enteral volumes </=60 m. L/kg/d, and were about to be treated with indomethacin or ibuprofen. Randomized at 6. 5 +/- 3. 9 days to receive "trophic" feeds ("feeding" group, n = 81: indomethacin 80%, ibuprofen 20%) or no feeds ("fasting/NPO" group, n = 96: indomethacin 75%, ibuprofen 25%) during the drug administration period. • Infants randomized to the "feeding" arm required fewer days to reach the study's feeding volume end point (120 m. L/kg/d). • Although the enteral feeding end point was reached at an earlier postnatal age, the age at which central venous lines were removed did not differ between the 2 groups. • There were no differences between the 2 groups in the incidence of infection, necrotizing enterocolitis, spontaneous intestinal perforation, or other neonatal morbidities. • •

ACID REDUCING AGENTS DO NOT WORK AND MAY BE DANGEROUS

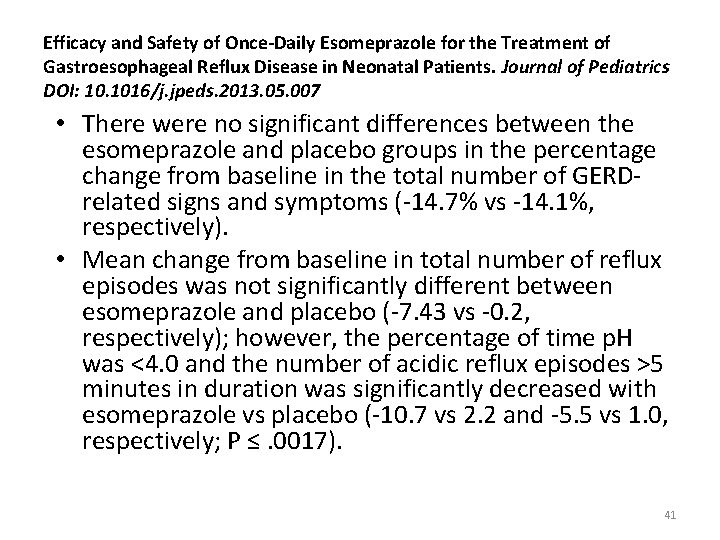
Efficacy and Safety of Once-Daily Esomeprazole for the Treatment of Gastroesophageal Reflux Disease in Neonatal Patients. Journal of Pediatrics DOI: 10. 1016/j. jpeds. 2013. 05. 007 • There were no significant differences between the esomeprazole and placebo groups in the percentage change from baseline in the total number of GERDrelated signs and symptoms (-14. 7% vs -14. 1%, respectively). • Mean change from baseline in total number of reflux episodes was not significantly different between esomeprazole and placebo (-7. 43 vs -0. 2, respectively); however, the percentage of time p. H was <4. 0 and the number of acidic reflux episodes >5 minutes in duration was significantly decreased with esomeprazole vs placebo (-10. 7 vs 2. 2 and -5. 5 vs 1. 0, respectively; P ≤. 0017). 41

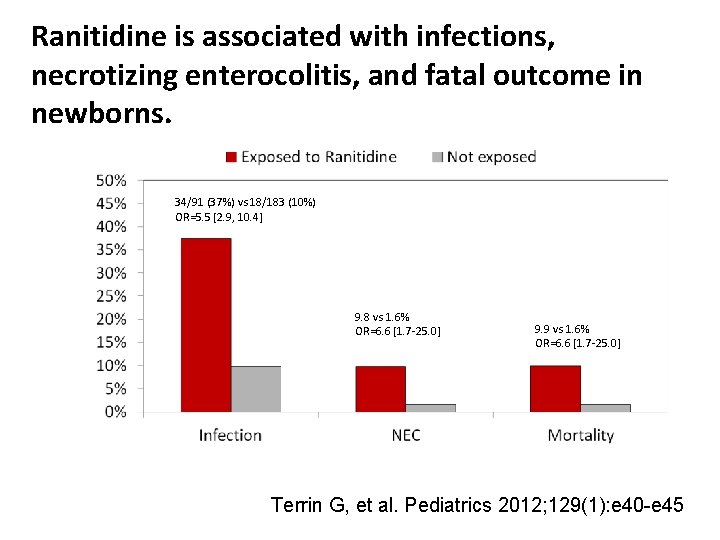
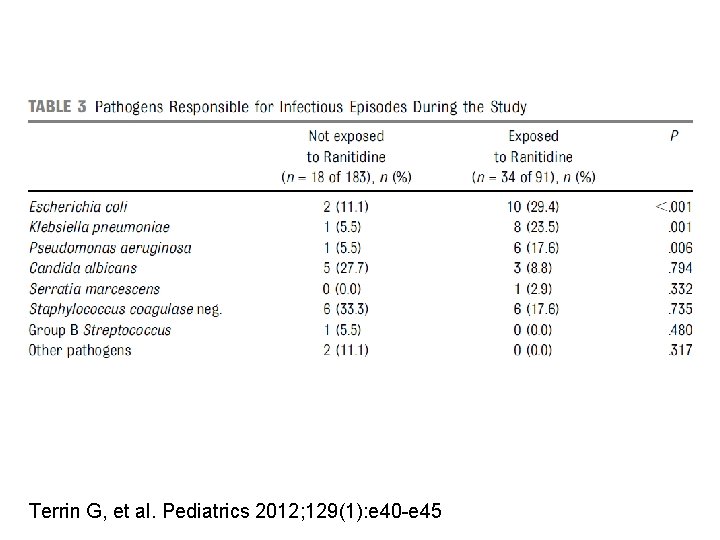
Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. 34/91 (37%) vs 18/183 (10%) OR=5. 5 [2. 9, 10. 4] 9. 8 vs 1. 6% OR=6. 6 [1. 7 -25. 0] 9. 9 vs 1. 6% OR=6. 6 [1. 7 -25. 0] Terrin G, et al. Pediatrics 2012; 129(1): e 40 -e 45

Terrin G, et al. Pediatrics 2012; 129(1): e 40 -e 45

Gupta RW, Tran L, Norori J, Ferris MJ, Eren AM, Taylor CM, et al. Histamine 2 receptor blockers alter the fecal microbiota in premature infants. J Pediatr Gastroenterol Nutr 2013 Apr; 56(4): 397 -400 • The objective of the present study was to test the hypothesis that histamine-2 receptor (H 2 -) blockers alter colonic bacterial colonization by analyzing and comparing the fecal microbiota in premature infants with and without H 2 -blocker therapy using sensitive molecular biological techniques. • Seventy-six premature infants </=1500 g or <34 weeks gestation were enrolled in this case-controlled, cross-sectional study. Stool samples were collected from 25 infants receiving H 2 -blockers and 51 babies who had never received them. • Proteobacteria and Firmicutes were the major phyla contributing to fecal microbial communities. • Microbial diversity was lower, relative abundance of Proteobacteria (primarily of the family Enterobacteriaceae) was increased, whereas that of Firmicutes was decreased in the stools of infants receiving H 2 -blockers compared with those who had never received them.

Antibiotic Stewardship

Prolonged Antibiotic Therapy for "Culture-Negative" Sepsis in Preterm Infants: It's Time to Stop! • Prolonged antibiotic therapy is more related to institutional decisions than severity of illness • Biological plausibility to explain some of these negative associations. – Antibiotic therapy affects gastrointestinal flora of preterm infants which can impact the development of NEC and late -onset sepsis, both of which can lead to death. – Elimination of commensal flora allows for colonization by multi-drug resistant organisms and fungi Cantey JB. J Pediatr. 2011; 159: 707 -708.

Association Of Initial Empirical Antibiotic Treatment With Necrotizing Enterocolitis And Death For Extremely Low Birth Weight Infants • Retrospective cohort analysis of ELBW infants admitted 1998 -2001. • Defined initial empirical antibiotic treatment duration as continuous days of antibiotic therapy started in the first 3 postnatal days with sterile culture results. • Prolonged empirical antibiotic therapy (≥ 5 days) • 4039 infants survived >5 days and included in study • Median therapy duration was 5 days (range: 1 -36 days); • 2147 infants (53%) received prolonged empirical therapy (center range: 27%-85%). • Multivariable analyses (adjusted for GA, Apgar score, race, center) prolonged therapy was associated with increased odds of necrotizing enterocolitis or death and death alone. Cotten, Pediatrics, 2009.

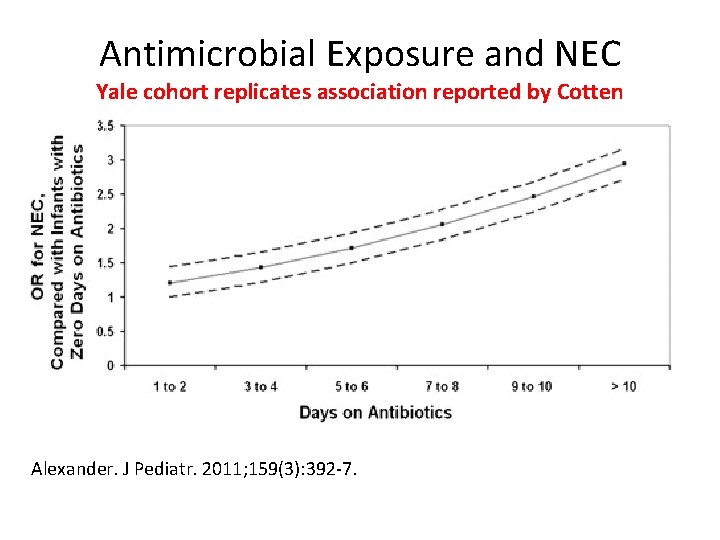
Antimicrobial Exposure and NEC Yale cohort replicates association reported by Cotten Alexander. J Pediatr. 2011; 159(3): 392 -7.

Probiotics

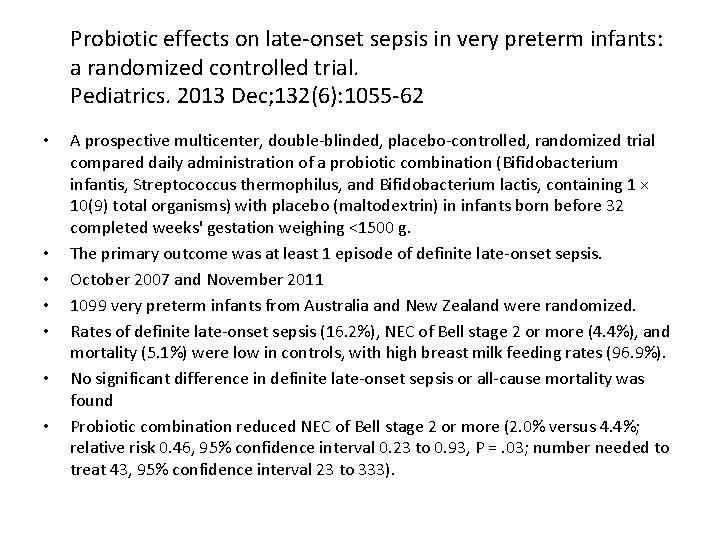
Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics. 2013 Dec; 132(6): 1055 -62 • • A prospective multicenter, double-blinded, placebo-controlled, randomized trial compared daily administration of a probiotic combination (Bifidobacterium infantis, Streptococcus thermophilus, and Bifidobacterium lactis, containing 1 × 10(9) total organisms) with placebo (maltodextrin) in infants born before 32 completed weeks' gestation weighing <1500 g. The primary outcome was at least 1 episode of definite late-onset sepsis. October 2007 and November 2011 1099 very preterm infants from Australia and New Zealand were randomized. Rates of definite late-onset sepsis (16. 2%), NEC of Bell stage 2 or more (4. 4%), and mortality (5. 1%) were low in controls, with high breast milk feeding rates (96. 9%). No significant difference in definite late-onset sepsis or all-cause mortality was found Probiotic combination reduced NEC of Bell stage 2 or more (2. 0% versus 4. 4%; relative risk 0. 46, 95% confidence interval 0. 23 to 0. 93, P =. 03; number needed to treat 43, 95% confidence interval 23 to 333).

William Tarnow-Mordi and Roger Soll. Probiotic Supplementation in Preterm Infants: It Is Time to Change Practice. The Journal of Pediatrics Volume 164, Issue 5 , Pages 959 -960, May 2014. • Probiotic supplementation in preterm infants is perhaps the best studied yet least used therapy in neonatal medicine. • All recently published meta-analyses have reported significant impacts on important clinical outcomes. • In the updated Cochrane meta-analysis, which includes 24 trials, there is a significant decrease in necrotizing enterocolitis (NEC) (relative risk 0. 43, 95% CI 0. 33 -0. 56; risk difference of 3%; and allcause mortality (RR 0. 65, 95% CI 0. 52 -0. 81). • Length of stay was 3 -4 days shorter. • There was no probiotic-related sepsis, although this is rarely reported outside trials. • Probiotics are still infrequently used in North America. In 2012, only 8%-9% of very low birth weight (VLBW) infants in the Vermont Oxford Network received probiotics.

Transfusions

Wallenstein MB, Arain YH, Birnie KL, Andrews J, Palma JP, Benitz WE et al. Red Blood Cell Transfusion Is Not Associated with Necrotizing Enterocolitis: A Review of Consecutive Transfusions in a Tertiary Neonatal Intensive Care Unit. J Pediatr 2014 • A retrospective cohort study was conducted for all infants weighing <1500 g who received at least 1 packed red blood cell transfusion between January 2008 and June 2013 in a tertiary neonatal intensive care unit. • The primary outcome was NEC, defined as Bell stage II or greater. • The temporal association of NEC and transfusion was assessed using multivariate Poisson regression. • The study sample included 414 very low birth weight infants who received 2889 consecutive red blood cell transfusions. Twenty-four infants (5. 8%) developed NEC. • Four cases of NEC occurred within 48 hours of a previous transfusion event. • Using multivariate Poisson regression, we did not find evidence of a temporal association between NEC and transfusion (P =. 32).

FEASIBILITY • How do we know that we can actually impact NEC on a national level? • Because we already have…

100, 000 babies Campaign… (credit to Dan Ellsbury & Robert Ursprung) • Enhance Nutrition: – Maximize breast milk use, use a standardized feeding protocol, and provide early protein • Improve Medication Use: – Optimize use of antenatal steroids, caffeine, and surfactant. Optimize antibiotic choice and exposure, decrease cephalosporin use, H-2 blocker use, and postnatal steroid use, standardize oxygen management • Optimize Central Line Use: – Standardized central line insertion process, standardized central line maintenance process, and minimize central line duration • Reduce Suboptimal Admission Temperatures: – Standardize initial thermal management

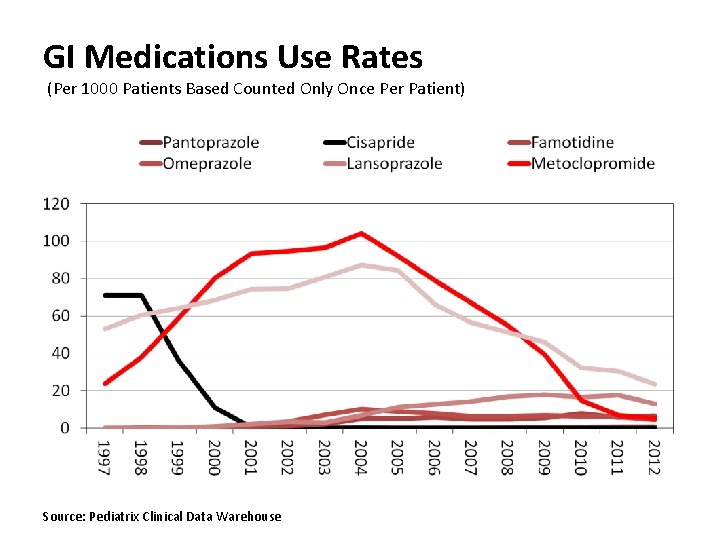
GI Medications Use Rates (Per 1000 Patients Based Counted Only Once Per Patient) Source: Pediatrix Clinical Data Warehouse



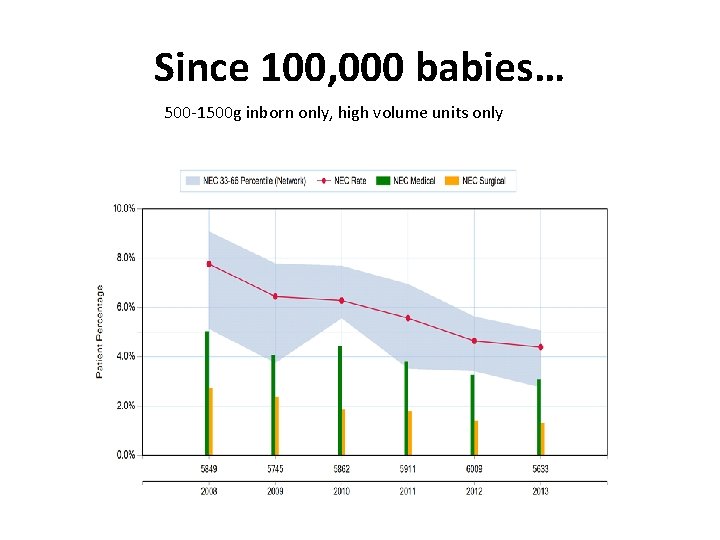
Since 100, 000 babies… 500 -1500 g inborn only, high volume units only

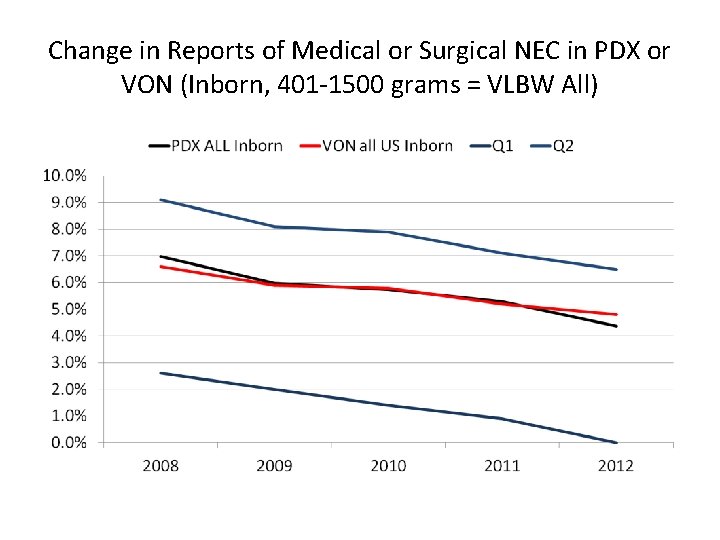
Change in Reports of Medical or Surgical NEC in PDX or VON (Inborn, 401 -1500 grams = VLBW All)

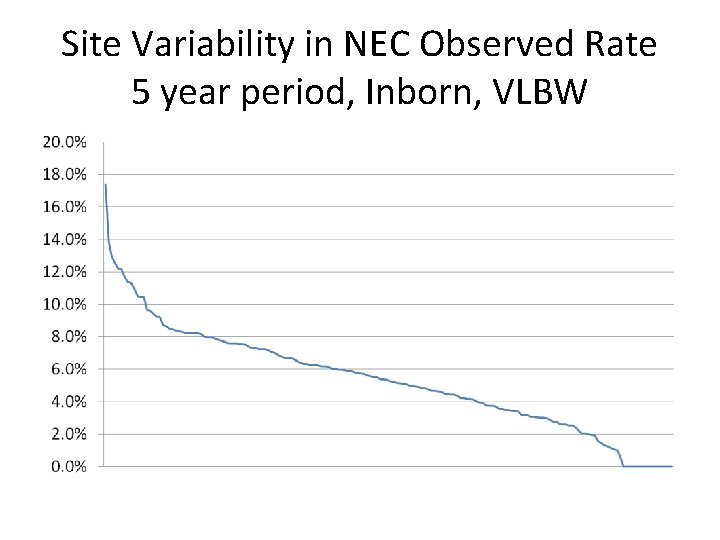
Site Variability in NEC Observed Rate 5 year period, Inborn, VLBW

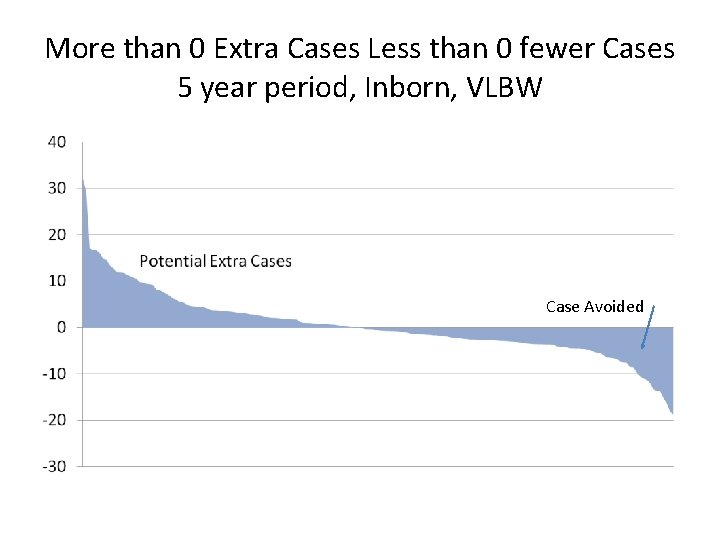
More than 0 Extra Cases Less than 0 fewer Cases 5 year period, Inborn, VLBW Case Avoided

Vaidyanathan Ganapathy et al. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatrics 2013, 13: 127 • Two hundred fifty NEC survivors (73 with surgical NEC) and 2, 909 matched controls were available for follow-up. • Medical NEC infants incurred significantly higher healthcare costs than matched controls between 6– 12 months of age (mean incremental cost = US$ 5, 112 per infant). • No significant difference in healthcare costs between medical NEC infants and matched controls was seen after 12 months. • Surgical NEC survivors incurred healthcare costs that were consistently higher than that of matched controls through 36 months of age. • The mean incremental healthcare costs of surgical NEC infants compared to matched controls between 6– 12, 12– 24 and 24– 36 months of age were US$ 18, 274, 14, 067 (p < 0. 01) and 8, 501 (p = 0. 06) per infant per six month period, respectively.

Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr 2010; 157: 751 -756. • Children who had surgical NEC unaccompanied by late bacteremia were at increased risk of psychomotor developmental indexes <70 (OR = 2. 7 [1. 2, 6. 4]), and children who had both surgical NEC and late bacteremia were at increased risk of diparetic cerebral palsy (OR = 8. 4 [1. 9, 39]) and microcephaly (OR = 9. 3 [2. 2, 40]). • In contrast, children who had medical NEC with or without late bacteremia were not at increased risk of any developmental dysfunction.

Keith Barrington Blog http: //neonatalresearch. org/2014/08/28/necrotizingenterocolitis-and-surgery/ • Hull MA, Fisher JG, Gutierrez IM, Jones BA, Kang KH, Kenny M, et al. Mortality and Management of Surgical Necrotizing Enterocolitis in Very Low Birth Weight Neonates: A Prospective Cohort Study. Journal of the American College of Surgeons. 2014; 218(6): 1148 -55. This data is drawn from the Vermont Oxford Network of infants with a diagnosis of NEC. About 50% of the 17, 000 infants required surgery, mortality prior to discharge was 30% among surgical cases, mortality among surgical cases was similar for the tiniest babies and for larger ones. Non-surgical NEC had a mortality that was greater among the smallest infants. The age at death is not reported, but prolonged hospitalization and delayed death do occur unfortunately in this group of babies.

Keith Barrington Blog http: //neonatalresearch. org/2014/08/28/necrotizingenterocolitis-and-surgery/ • • Murthy K, Yanowitz TD, Di. Geronimo R, Dykes FD, Zaniletti I, Sharma J, et al. Shortterm outcomes for preterm infants with surgical necrotizing enterocolitis. J Perinatol. 2014. And from the Children’s hospitals neonatal consortium, a report of over 700 cases of surgical NEC, the babies were outborn (as it was from children’s hospitals) and were transferred for surgery. The study shows a hospital mortality of about 37% for the less mature babies (<28 weeks), and a little lower for the larger ones, 30%. Two additional outcomes, the frequency of short bowel syndrome was high at about 25% (it was defined as needing TPN for more than 90 days), but all except 2 of them were able to go home without TPN: Hospital stay tended to be very long for survivors, with a median of over 100 days, and a upper third quartile for the immature babies of 168 days. In other words a quarter of surgical NEC babies, <28 weeks, are hospitalised for 6 months or more. The total number of surgical procedures was over 2, 800, or about 4 per patient, some were re-anastamosis of ostomies, only 124 had a single laparotomy.