CAN ADHERENCE BE IMPROVED Status of Adherence Intervention

CAN ADHERENCE BE IMPROVED?
Status of Adherence Intervention Studies t To Medication t To Exercise t To Diet

19 Adherence Intervention Studies u u u Randomized Control Group Assessment of Adherence Assessment of Outcome 6 month Follow Up Haynes, R. B. , Montague, P. , Oliver, T. , Mc. Kibbon, K. A. , Brouwers, M. C. , & Kanani, R. (2001). Interventions for helping patients to follow prescriptions for medications. [Systematic Review] Cochrane Consumers & Communication Group Cochrane Database of Systematic Reviews.

19 Adherence Intervention Studies All Use Self - Report 1 Study addresses Remediation u Education/Counseling/Behavioral u All Strategies Address Single Regimen/Disease
Characteristics of Successful Interventions t Educational/Behavioral t Multicomponent t Long-Term (from Haynes, 1996)

Adherence Monitoring as Intervention t Use of Electronically Monitored Data as Feedback t Improved Blood Pressure Control 1 Improved Blood Pressure Management t Reduction in Seizures 2 Improved Adherence 1 Bertholet et al, 2000 2 Schneider et al, 2000
Summary of Interventions t Self-Monitoring t Education t Counseling t Social Support t Positive Reinforcement t Self-Efficacy Enhancement t Cuing t Behavioral Intervention t Verbal Persuasion t Electronic Monitoring/Feedback
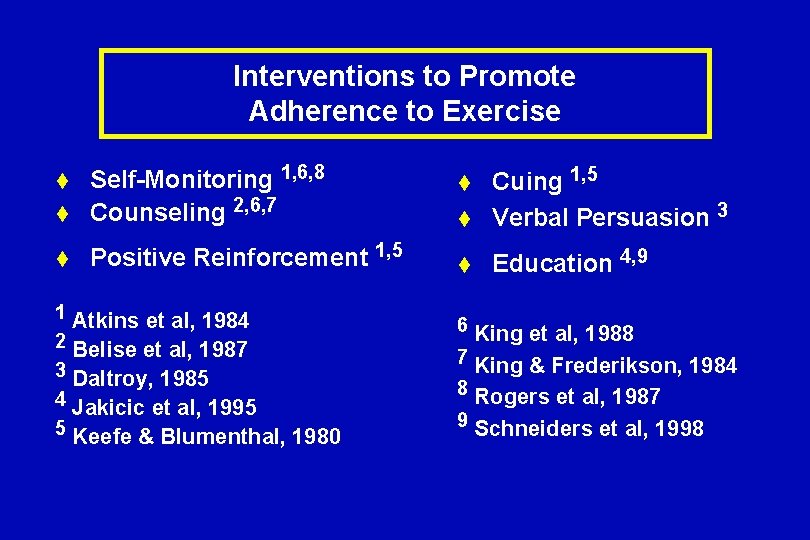
Interventions to Promote Adherence to Exercise t Self-Monitoring 1, 6, 8 Counseling 2, 6, 7 t Positive Reinforcement 1, 5 t 1 2 3 4 5 Atkins et al, 1984 Belise et al, 1987 Daltroy, 1985 Jakicic et al, 1995 Keefe & Blumenthal, 1980 t Cuing 1, 5 Verbal Persuasion 3 t Education 4, 9 t 6 King et al, 1988 7 King & Frederikson, 1984 8 Rogers et al, 1987 9 Schneiders et al, 1998

Interventions to Promote Adherence to Dietary Regimen t Counseling 3, 4, 8 1, 2, 6 t Social Support t Self-Efficacy Enhancement 6 1 2 3 4 5 Barnard et al, 1992 Borbjerb et al, 1995 Dolecek et al, 1986 Glueck et al, 1986 Karvetti, 1981 t Education 5, 7 t Behavioral Intervention 9 6 Mc. Cann et al, 1988 7 Mojonnier et al, 1980 8 Simkin-Silverman et al, 1995 9 Wing & Anglen, 1996

Summary t Interventions are not targeted to patient adherence patterns or to patient-reported reasons for poor adherence t Outcome t Very measures are not reliable or accurate few RCT’s have been reported

3 Randomized Controlled Studies Designed to Examine Strategies to Improve Compliance Study 1. An intervention study designed to improve poor adherers - asymptomatic condition Study 2. An intervention study with poor compliers symptomatic condition Study 3. Adherence in clinical trials - an induction study

An Intervention Study Designed to Improve Poor Compliers Purpose: To evaluate a multicomponent behavioral strategy designed to improve compliance among poor compliers Setting: Multi-center randomized controlled clinical trial designed the cholesterol hypothesis * Coronary Primary Prevention Trial to test
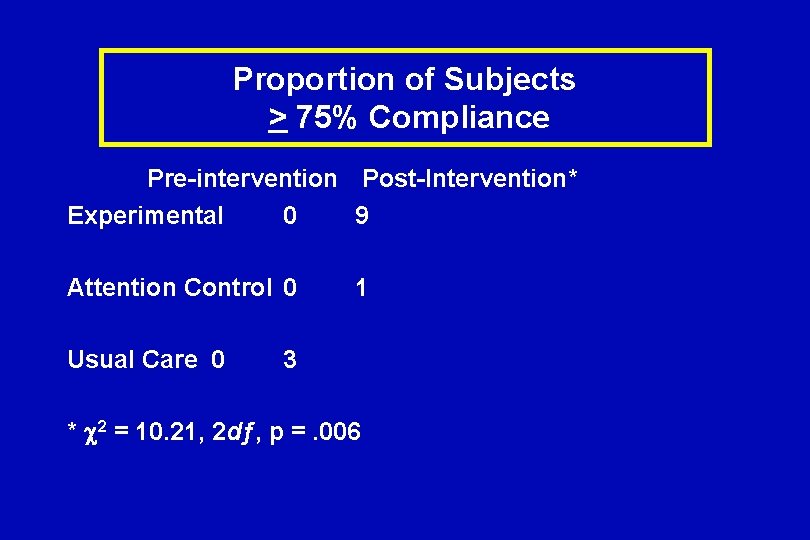
Proportion of Subjects > 75% Compliance Pre-intervention Post-Intervention* Experimental 0 9 Attention Control 0 Usual Care 0 1 3 * 2 = 10. 21, 2 dƒ, p =. 006
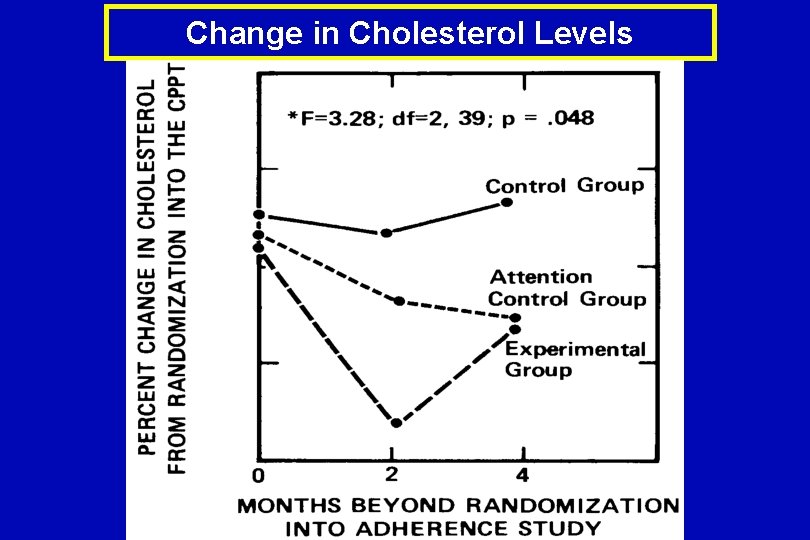
Change in Cholesterol Levels
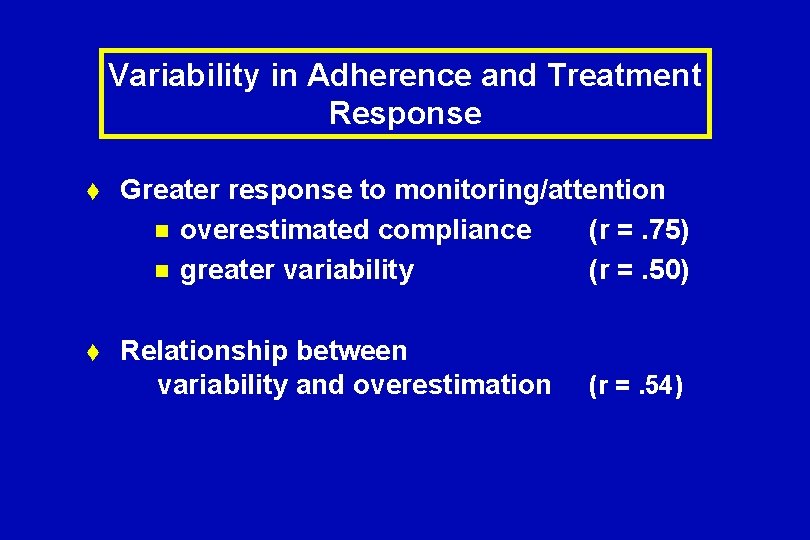
Variability in Adherence and Treatment Response t Greater response to monitoring/attention n overestimated compliance (r =. 75) n greater variability (r =. 50) t Relationship between variability and overestimation (r =. 54)

An Intervention Study Designed to Improve Poor Adherers. RAC-1 Purpose: To evaluate a series of behavioral/problem solving interventions to improve poor adherence Setting: Specialty practice sites
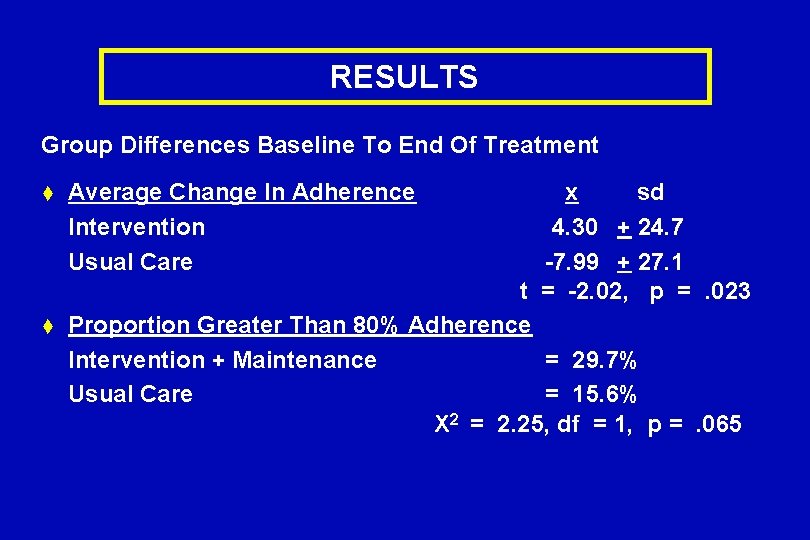
RESULTS Group Differences Baseline To End Of Treatment t t Average Change In Adherence Intervention Usual Care x sd 4. 30 + 24. 7 -7. 99 + 27. 1 t = -2. 02, p =. 023 Proportion Greater Than 80% Adherence Intervention + Maintenance = 29. 7% Usual Care = 15. 6% X 2 = 2. 25, df = 1, p =. 065
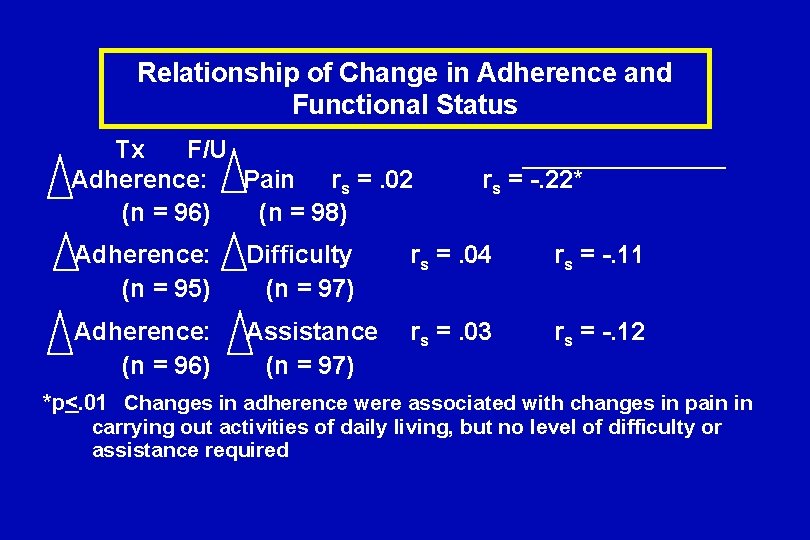
Relationship of Change in Adherence and Functional Status Tx F/U Adherence: Pain rs =. 02 (n = 96) (n = 98) rs = -. 22* Adherence: (n = 95) Difficulty (n = 97) rs =. 04 rs = -. 11 Adherence: (n = 96) Assistance (n = 97) rs =. 03 rs = -. 12 *p<. 01 Changes in adherence were associated with changes in pain in carrying out activities of daily living, but no level of difficulty or assistance required
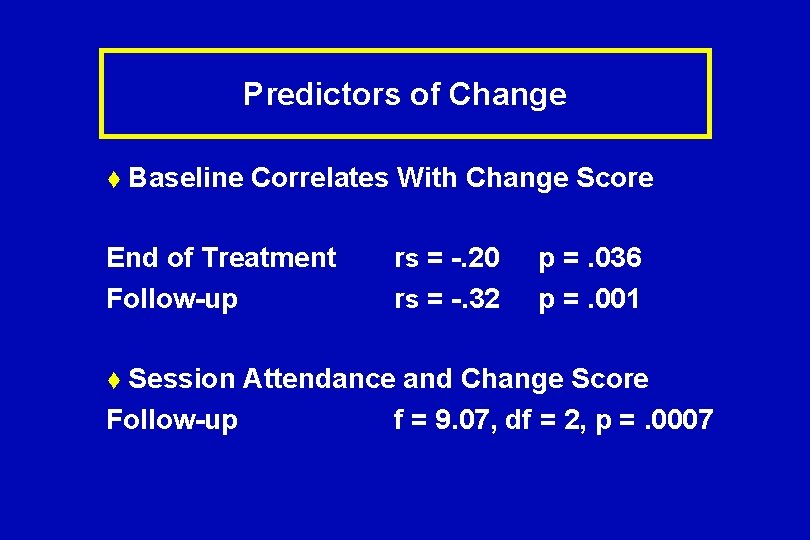
Predictors of Change t Baseline Correlates With Change Score End of Treatment Follow-up t Session rs = -. 20 rs = -. 32 p =. 036 p =. 001 Attendance and Change Score Follow-up f = 9. 07, df = 2, p =. 0007
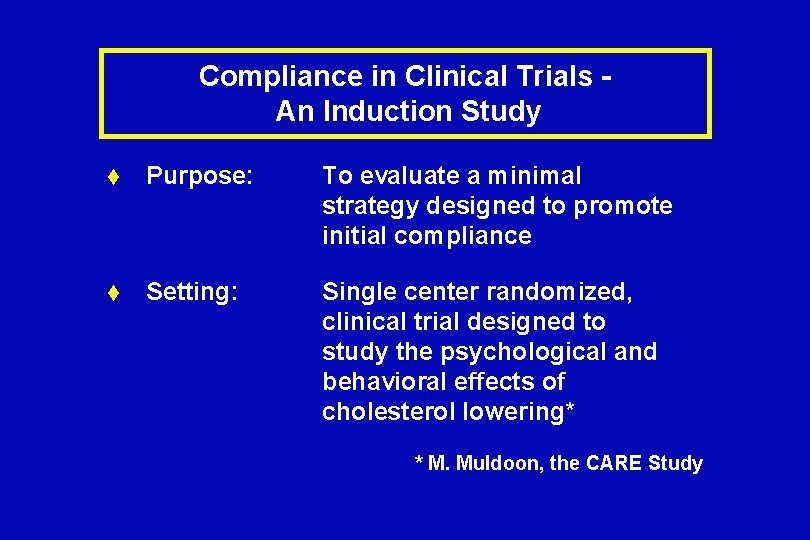
Compliance in Clinical Trials An Induction Study t Purpose: To evaluate a minimal strategy designed to promote initial compliance t Setting: Single center randomized, clinical trial designed to study the psychological and behavioral effects of cholesterol lowering* * M. Muldoon, the CARE Study
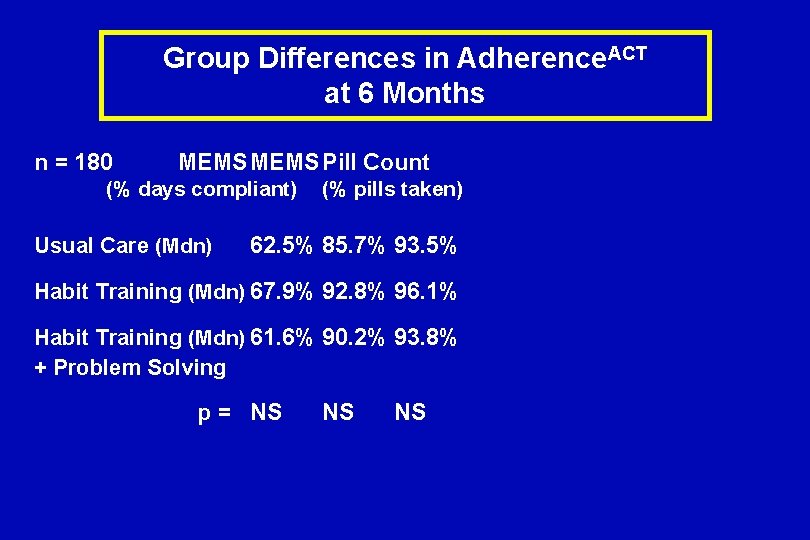
Group Differences in Adherence. ACT at 6 Months n = 180 MEMS Pill Count (% days compliant) Usual Care (Mdn) (% pills taken) 62. 5% 85. 7% 93. 5% Habit Training (Mdn) 67. 9% 92. 8% 96. 1% Habit Training (Mdn) 61. 6% 90. 2% 93. 8% + Problem Solving p = NS NS NS

Summary t Poor n n t Few Adherence is: Wide Spread Costly Hard to Identify Difficult to Predict Who Does Not Adhere Studies Point to Interventions

Summary t Individuals vary in dosing adherence t Measures to identify poor adherence need to be sensitive to dosing patterns t Minimal intervention does not appear to improve long-term adherence t Adherence can be improved with intensive interventions t Improving adherence positively impacts clinical outcomes

Recommendations t Address individual adherence patterns in clinical and research setting t Take careful account of method of assessment in interpretation of adherence data t Design/evaluate adherence interventions

Any Questions? Thank You!
- Slides: 25