Calorimetry Measurement of Enthalpy Change Specific heat capacity


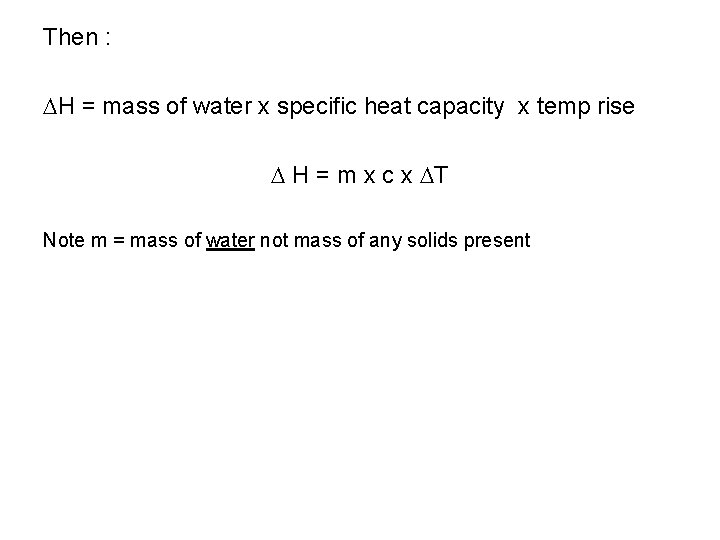






- Slides: 13

Calorimetry Measurement of Enthalpy Change

Specific heat capacity is the amount of heat needed to raise the temperature of 1 g of substance by 1 K Specific heat capacity of water = 4. 18 KJ kg-1 K-1 or 4. 18 J g -1 K 1 Be careful with the units it could also be quoted as KJ g-1 K-1 Ensure you use the correct units in your calculation! To measure the heat released in a process we arrange for the heat to be transferred to a substance (usually water) then measure the temperature rise.
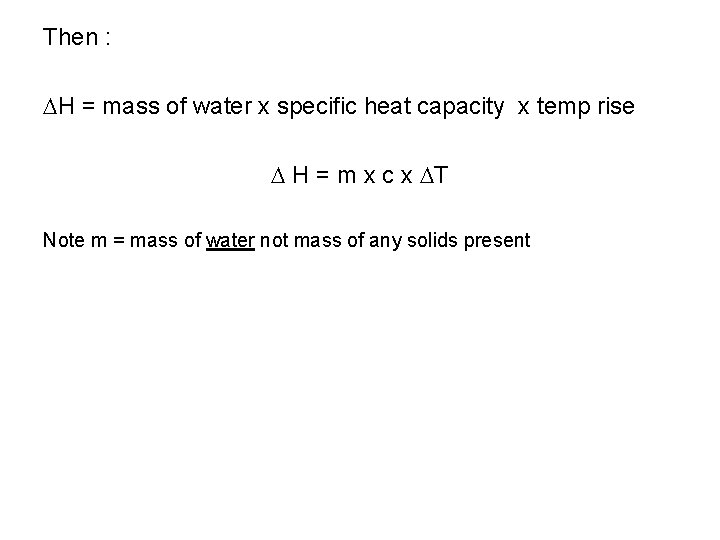
Then : H = mass of water x specific heat capacity x temp rise H = m x c x T Note m = mass of water not mass of any solids present

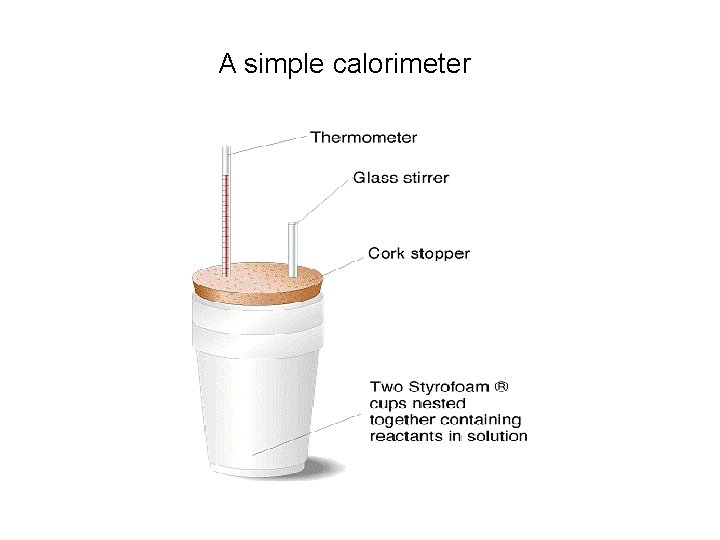
Measuring Enthalpy Changes in the Laboratory Apparatus needed An insulated container to serve as a calorimeter A thermometer A balance Volumetric appaaratus (e. g burette, pipette, measuring cylinder)

A simple calorimeter


Some general steps in the procedure 1) Allow a known mass or volume of reactants to reach the temperature of the surroundings 2) Thoroughly mix the reactants and record the highest or lowest temperature reached 3) Determine the temperature change for the reaction 4) Calculate the enthalpy change for the reaction For a given mass (m kg) of reacting substance the heat energy released is calculated using the equation Heat = m x c x T

Assumptions and Errors • For aqueous solutions we assume that 1 ml has a mass of 1 g and that for dilute solutions the specific heat capacity is the same as that of water. • These assumptions will give minor errors in our calculations • The biggest error will be heat lost to the surroundings (i. e to thermometer, the surrounding air and the container) This can be minimised by the use of an adequately insulated calorimeter

Excess powdered zinc was added to 100 ml of 0. 2 mol/L copper (II) sulphate solution. A temperature rise of 10 o. C was recorded. Find the enthalpy change for the reaction. H = m x c x T H = 100 g x 4. 18 KJ kg-1 K-1 x 10 KJ 1000 = 4. 180 KJ This is for the no of moles of Cu. SO 4 used in the experiment No of moles of Cu. SO 4 = 0. 2 x 100 = 0. 02 moles 1000 H = 4. 180 = 209 KJ mol-1 0. 02 The reaction is exothermic so we need to put in a negative sign Hr = - 209 KJ mol-1 Note We do not use the standard sign as standard conditions were not used.

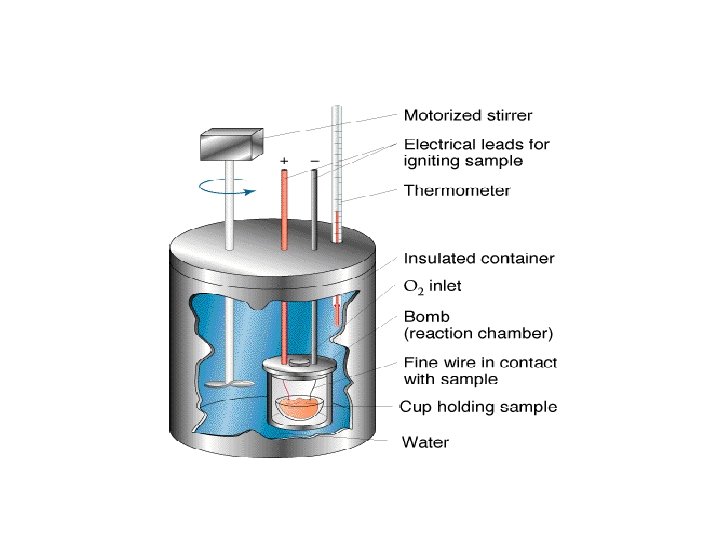
Combustion To find the heat of combustion of a substance a known mass of the substance is burned, the heat released transferred to water and the enthalpy change found as before

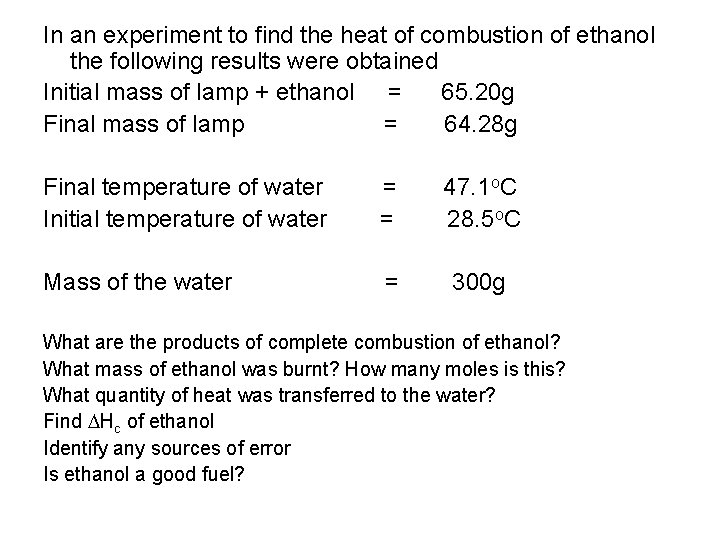
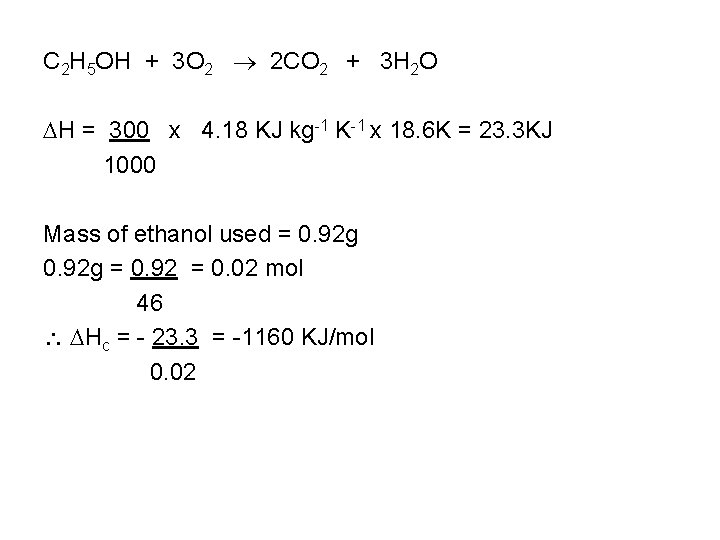
In an experiment to find the heat of combustion of ethanol the following results were obtained Initial mass of lamp + ethanol = 65. 20 g Final mass of lamp = 64. 28 g Final temperature of water Initial temperature of water = = 47. 1 o. C 28. 5 o. C Mass of the water = 300 g What are the products of complete combustion of ethanol? What mass of ethanol was burnt? How many moles is this? What quantity of heat was transferred to the water? Find Hc of ethanol Identify any sources of error Is ethanol a good fuel?

C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O H = 300 x 4. 18 KJ kg-1 K-1 x 18. 6 K = 23. 3 KJ 1000 Mass of ethanol used = 0. 92 g = 0. 92 = 0. 02 mol 46 Hc = - 23. 3 = -1160 KJ/mol 0. 02

Errors Heat lost to surroundings (air, can thermometer) Errors in measuring temperature change (unavoidable error in reading thermometer) Errors in measuring masses (unavoidable error in reading balance) The enthalpy change of combustion is high ethanol is a good fuel