Calorimetry EQ Describe the parts and each parts

Calorimetry EQ: Describe the parts and each part’s function in a calorimeter?
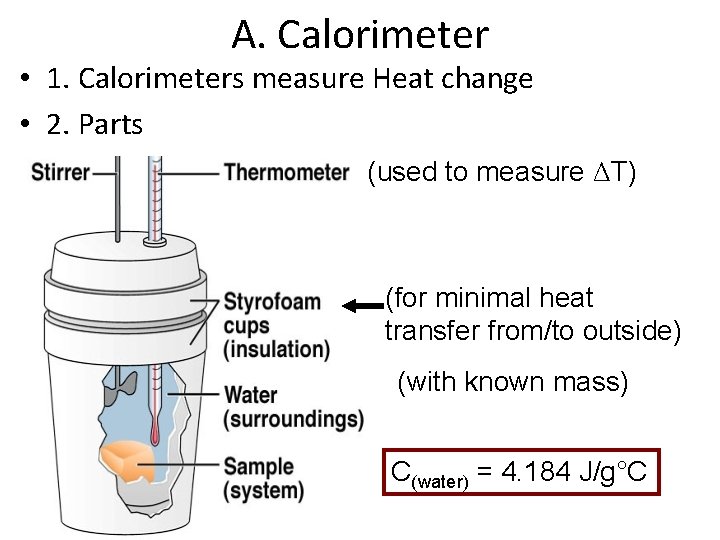
A. Calorimeter • 1. Calorimeters measure Heat change • 2. Parts (used to measure ΔT) (for minimal heat transfer from/to outside) (with known mass) C(water) = 4. 184 J/g°C
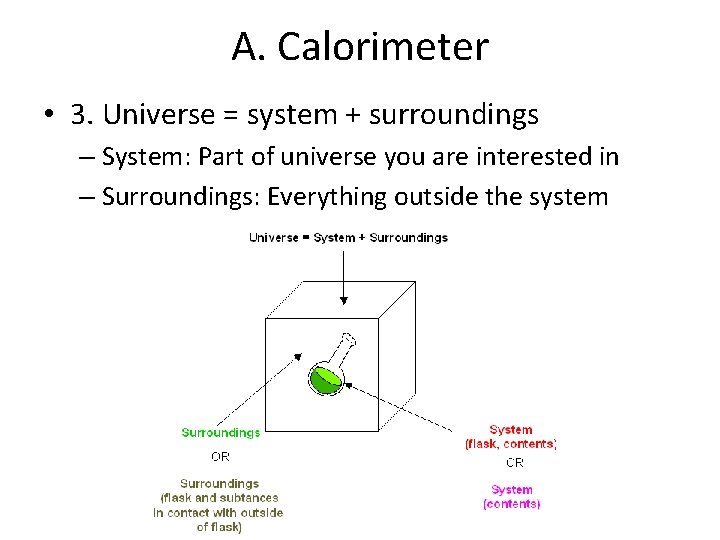
A. Calorimeter • 3. Universe = system + surroundings – System: Part of universe you are interested in – Surroundings: Everything outside the system
A. Calorimeter • 4. Calorimetry depends of the Law of conservation of energy – Heat lost or gained by the reaction or metal = Heat lost or gained by the calorimeter (water) • 5. Heat absorbed= +q • Heat released = -q
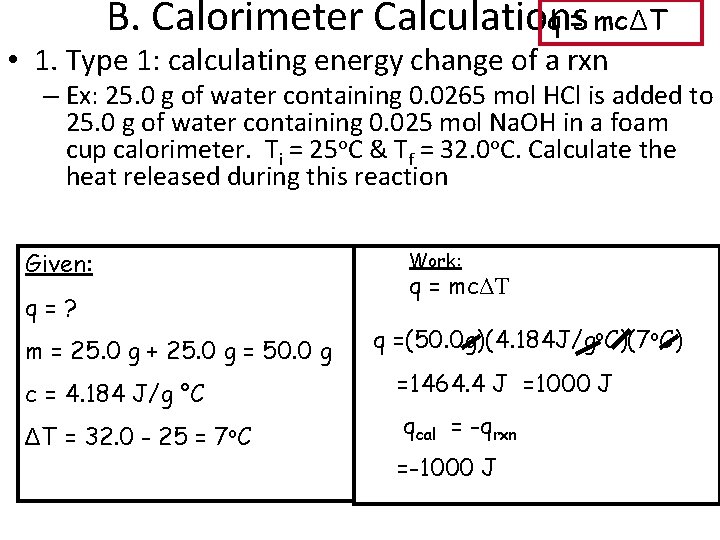
B. Calorimeter Calculations q = mcΔT • 1. Type 1: calculating energy change of a rxn – Ex: 25. 0 g of water containing 0. 0265 mol HCl is added to 25. 0 g of water containing 0. 025 mol Na. OH in a foam cup calorimeter. Ti = 25 o. C & Tf = 32. 0 o. C. Calculate the heat released during this reaction Given: q=? m = 25. 0 g + 25. 0 g = 50. 0 g c = 4. 184 J/g °C ΔT = 32. 0 - 25 = 7 o. C Work: q = mcΔT q =(50. 0 g)(4. 184 J/go. C)(7 o. C) =1464. 4 J =1000 J qcal = -qrxn =-1000 J
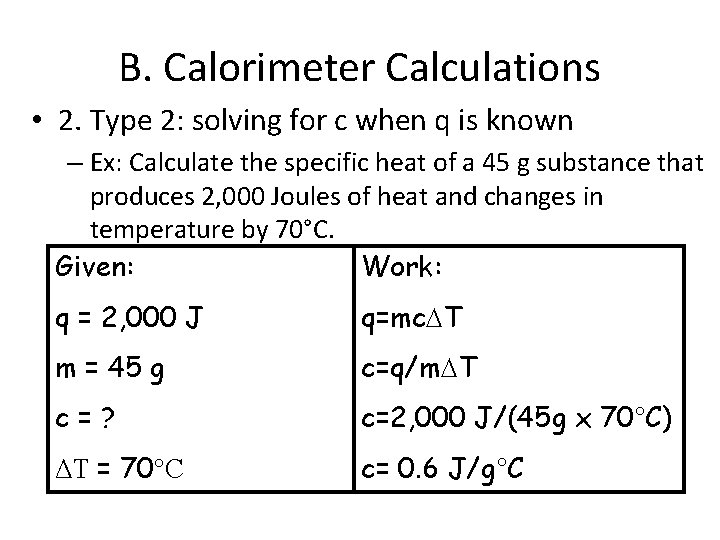
B. Calorimeter Calculations • 2. Type 2: solving for c when q is known – Ex: Calculate the specific heat of a 45 g substance that produces 2, 000 Joules of heat and changes in temperature by 70°C. Given: Work: q = 2, 000 J q=mc T m = 45 g c=q/m T c=? c=2, 000 J/(45 g x 70°C) ΔT = 70°C c= 0. 6 J/g°C
B. Calorimeter Calculations • 3. Type 3: solving for c when q is unknown – Ex: A 50. 0 g piece of metal is heated to 115. 0°C and is placed into a calorimeter with 125 g of water whose initial temperature was 25. 6°C. Both the water and the metal have a final temperature of 29. 3°C. What is the specific heat of the metal?
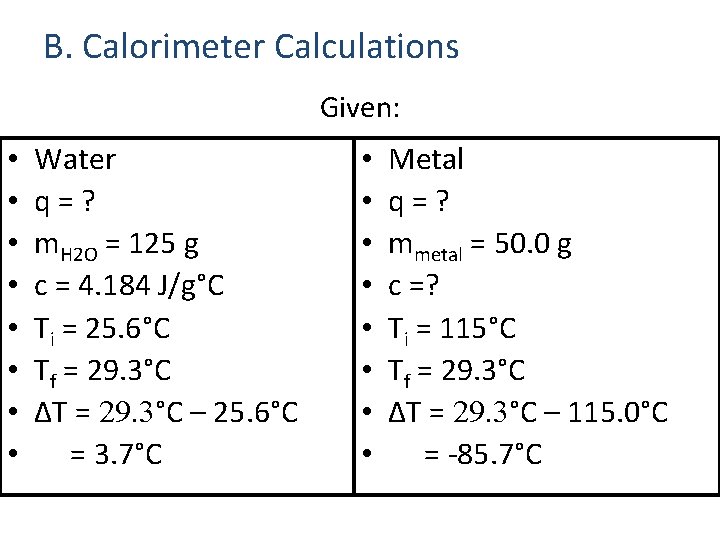
B. Calorimeter Calculations Given: • • Water q=? m. H 2 O = 125 g c = 4. 184 J/g°C Ti = 25. 6°C Tf = 29. 3°C ΔT = 29. 3°C – 25. 6°C = 3. 7°C • • Metal q=? mmetal = 50. 0 g c =? Ti = 115°C Tf = 29. 3°C ΔT = 29. 3°C – 115. 0°C = -85. 7°C
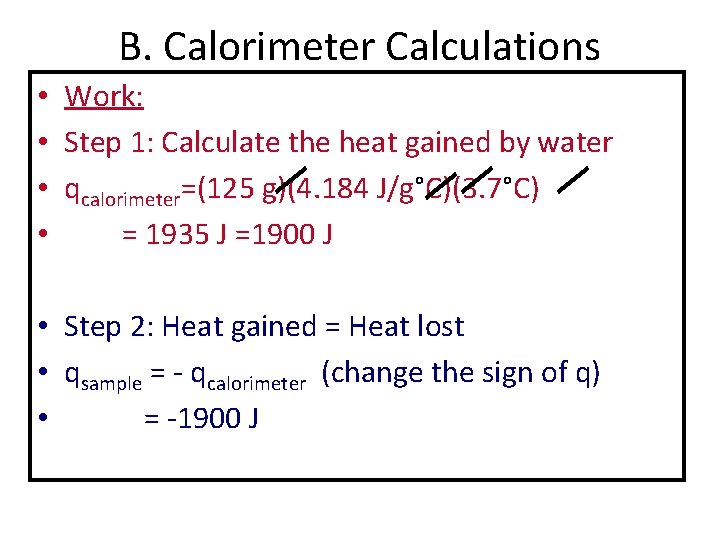
B. Calorimeter Calculations • Work: • Step 1: Calculate the heat gained by water • qcalorimeter=(125 g)(4. 184 J/g°C)(3. 7°C) • = 1935 J =1900 J • Step 2: Heat gained = Heat lost • qsample = - qcalorimeter (change the sign of q) • = -1900 J

B. Calorimeter calculations • Step 3: Determine Specific heat for metal • q = mcΔT • c=q = -1900 J = 0. 44 J/g°C mΔT (50. 0 g · -85. 7°C)
- Slides: 10