Calorimetry Calorimetry measurement of heat flow Calorimeter apparatus






- Slides: 9

Calorimetry

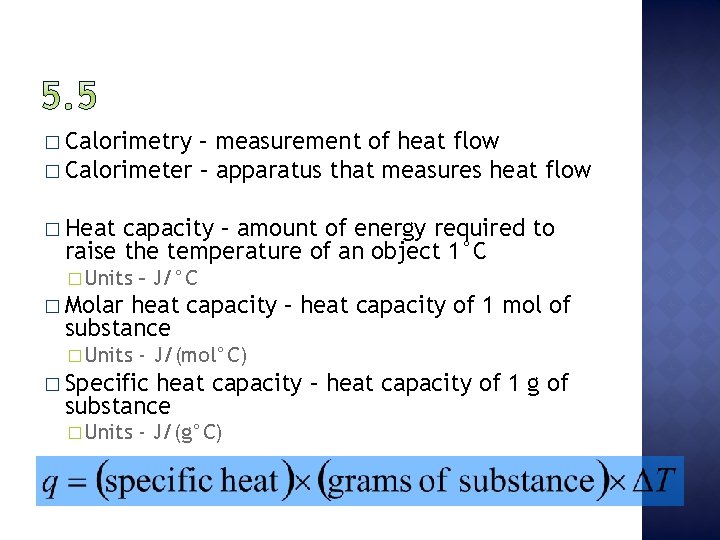
� Calorimetry – measurement of heat flow � Calorimeter – apparatus that measures heat flow � Heat capacity – amount of energy required to raise the temperature of an object 1°C � Units – J/°C � Molar heat capacity – heat capacity of 1 mol of substance � Units - J/(mol°C) � Specific heat capacity – heat capacity of 1 g of substance � Units - J/(g°C)

� How much heat is needed to warm 250 g of water from 22°C to 98°C? The specific heat of water is 4. 184 J/g°C �q = m. C∆T �q = (250 g)(4. 184 J/g°C)(98 -22°C) �q = 79496 J = 79000 J or 79 k. J

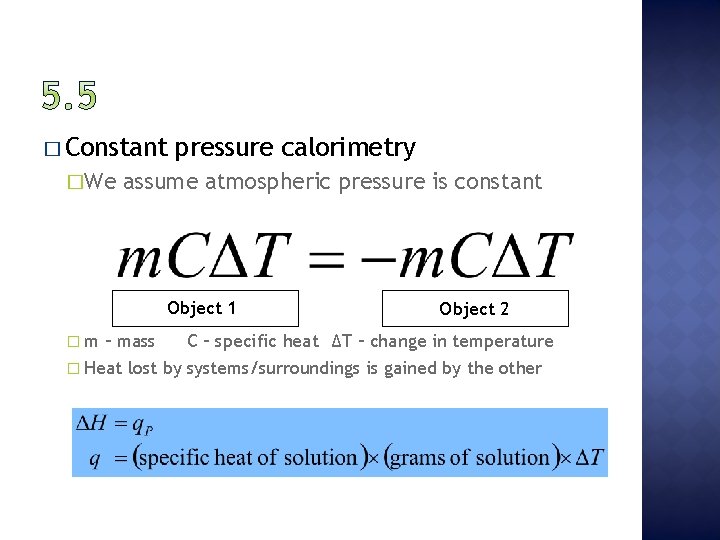
� Constant �We pressure calorimetry assume atmospheric pressure is constant Object 1 �m Object 2 – mass C – specific heat ∆T – change in temperature � Heat lost by systems/surroundings is gained by the other

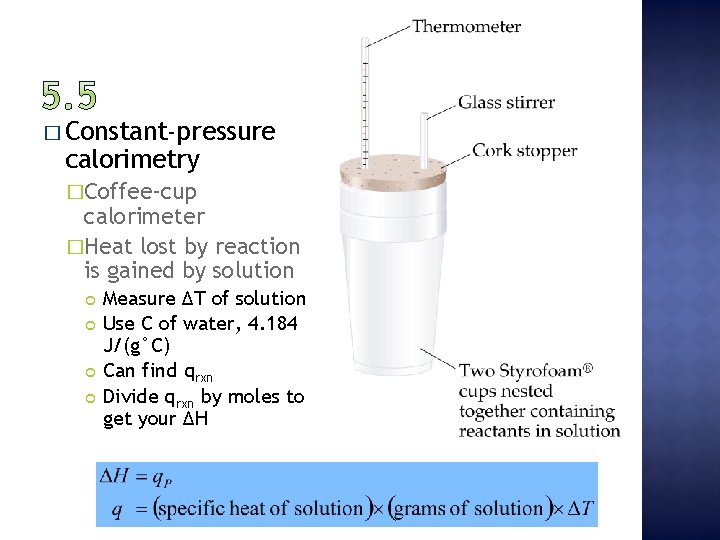
� Constant-pressure calorimetry �Coffee-cup calorimeter �Heat lost by reaction is gained by solution Measure ∆T of solution Use C of water, 4. 184 J/(g°C) Can find qrxn Divide qrxn by moles to get your ∆H

� You �An try! unknown metal is to be analyzed for specifc heat. A 12. 84 g piece of the metal is placed in boiling water at 101. 5°C until it reached the same temperature. The metal is then removed from the boiling water and placed in a Styrofoam cup containing 59. 92 g of water at 23. 2°C. The final temperature of the water was 26. 1°C. What is the specific heat of the metal sample, in J/(g°C)?

� You try! � An unknown metal is to be analyzed for specific heat. A 12. 84 g piece of the metal is placed in boiling water at 101. 5°C until it reached the same temperature. The metal is then removed from the boiling water and placed in a Styrofoam cup containing 59. 92 g of water at 23. 2°C. The final temperature of the water was 26. 1°C. What is the specific heat of the metal sample, in J/(g°C)? �mwater. Cwater∆Twater � = -mmetal. Cmetal∆Tmetal (59. 92 g)(4. 184 J/g°C)(26. 1 -23. 2°C) = -(12. 84 g)(Cmetal)(26. 1 -101. 5°C) � 727. 045312 J = 965. 136 g°C (Cmetal) � 0. 753308665 J/(g°C) = Cmetal �Cmetal = 0. 75 J/(g°C)

� You try! � 25. 0 m. L of 0. 100 M Na. OH is mixed with 25. 0 m. L of 0. 100 M HNO 3, and the temperature of the mixture increases from 23. 1°C to 49. 8°C. Calculate ∆H for this reaction in k. J/mol. Assume that the specific heat of each solution is equal to the specific heat of water, 4. 184 J/(g°C), and their densities are 1. 00 g/m. L.

� You try! � 25. 0 m. L of 0. 100 M Na. OH is mixed with 25. 0 m. L of 0. 100 M HNO 3, and the temperature of the mixture increases from 23. 1°C to 49. 8°C. Calculate ∆H for this reaction in k. J/mol. Assume that the specific heat of each solution is equal to the specific heat of water, 4. 184 J/(g°C), and their densities are 1. 00 g/m. L. � qrxn = masssolution. Csolution∆Tsolution � qrxn = (50. 0 g)(4. 184 J/g°C)(49. 8 -23. 1°C) � qrxn = 5585. 64 J = 5. 58564 k. J � ∆H = 5. 58564 k. J ÷ x mol We have 0. 0025 mol of Na. OH and HNO 3 ∆H = 5. 58564 k. J ÷ 0. 0025 mol ∆H = 2234. 256 k. J/mol = 2230 k. J/mol

















