Calculating Heat Specific Heat l l Amount of







- Slides: 11

Calculating Heat

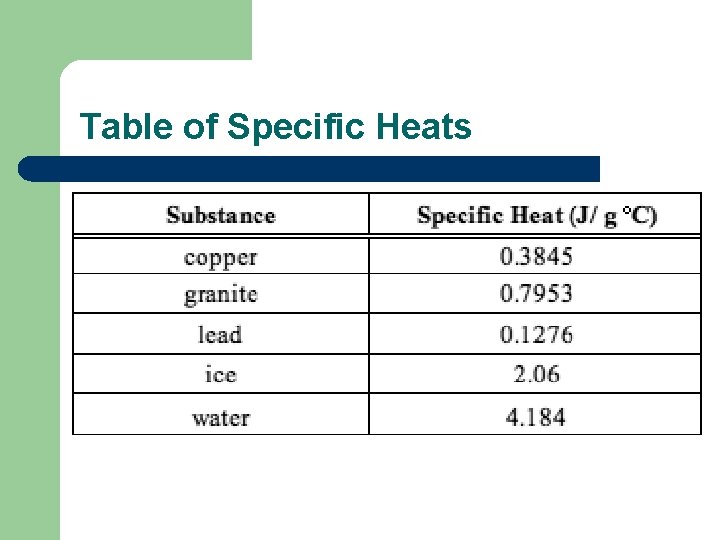
Specific Heat l l Amount of heat energy needed to raise the temp of 1 ml of a substance 1°C For water the specific heat is 4. 19 J/g °C, but it is differenct for different substances It takes 4. 19 J to raise the temp of 1 g of water 1 °C Every substance has a different specific heat

Table of Specific Heats

Calculating Heat When Substance in One Phase l l Heat released or absorbed can be calculated by multiplying three factors Heat = specific heat x mass x temp change q = c x m x Δt

Practice l l The specific heat of water is 4. 19 J/ g C. How much heat is needed to warm 350 g of water from 25 C to 75 C? If 178 J of heat is needed to increase the temperature of a 20 g piece of copper metal from 25 C to 48 C, what is the specific heat of copper?

Heat and Phase Change (Evaporation) l l l Heat of vaporization ( Hvao) energy required to change one gram of a substance from liquid to gas. For water 2260 J/g Q = Hvao m

Practice l l The heat of vaporization of water is 2260 J/g. How much heat must be supplied to evaporate 50 g of water? How much heat is required to evaporate 150. g of a substance at its boiling point if it has a Hvap= 987 J/g?

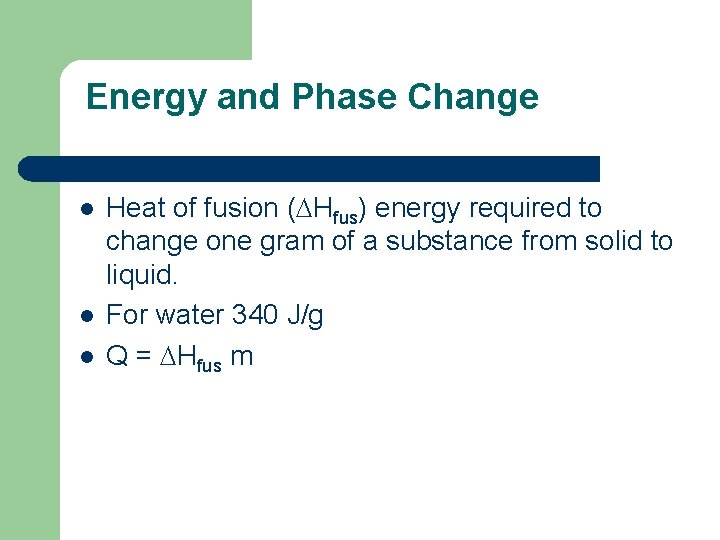
Energy and Phase Change l l l Heat of fusion ( Hfus) energy required to change one gram of a substance from solid to liquid. For water 340 J/g Q = Hfus m

Examples l l The heat of fusion of ice at 0°C is 340 J/g. How much heat is needed to change 75 g of ice at 0°C to a liquid at the same temperature? The heat of fusion of water at 0°C is 340 J/g. How much heat is released when 250 g of water changes to ice at 0°C?


Practice l l How much heat is needed to change 100 g of water at 50 C to steam at 120 C? The specific heat of water is 4. 19 J/g C, the specific heat of steam is 1. 7 J/g C, and the heat of vaporization of water is 2260 J/g. How much heat must be released as 50 g of water at 25 C to ice at -10 C? The specific heat of water is 4. 19 J/g C, the specific heat of ice is 2. 1 J/g C, and the heat of fusion fro water is 340 J/g.