Calculating Heat During Change of Phase Heat Added












- Slides: 12

Calculating Heat During Change of Phase Heat Added (J)

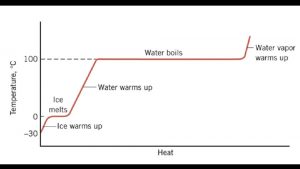
Heat in Changes of STATE p p p Heating and Cooling Curve LABEL: *physical states (solid, liquid, gas) *heat of fusion *heat of vaporization *boiling point *melting point (similar to pg. 523)

As a substance heats up (or cools down) each phase & phase change needs to be calculated separately. p Review n Specific heat is the amount of heat that must be added to a stated mass of a substance to raise its temperature by 1°C, with NO change in state. p. Specific heat of water = 4. 184 J/g°C p. Specific heat of ice =2. 09 J/g°C p. Specific heat of steam = 2. 03 J/g°C

Need to calculate heat for the WHOLE process of changing physical state p NEW CHANGING STATE requires “heat of vaporization” and “heat of fusion” n p qvap = m. Hvap Heat of vaporization = 2260 J/g n p Heat of vaporization = amount of heat that must be added to 1 g of a liquid at its boiling point to convert it to vapor with NO temp. change Heat of fusion = amount of heat needed to melt 1 g of a solid at its melting point qfus = m. Hfus Heat of fusion = 334 J/g

Values ***WRITE ON YOUR HEATING/COOLING CURVE Specific heat of water = 4. 184 J/g°C p Specific heat of ice =2. 09 J/g°C p Specific heat of steam = 2. 03 J/g°C p Heat of fusion = 334 J/g p Heat of vaporization = 2260 J/g p

USE YOUR HEATING/COOLING CURVE to help guide your calculations 1. How much heat is released by 250. 0 g of water as it cools from 85. 0 °C to 40. 0 °C? Calculation is within the liquid phase of water (no phase changes) q = mcΔT q = (250. 0 g)(4. 184 J/g°C)(40. 0°C - 85. 0°C) q = - 47070 J = (3 sig figs) - 47100 J

Example 2 - How much heat energy is required to bring 135. 5 g of water at 55. 0 °C to its boiling point and then vaporize it? p THINK!!! HOW MANY STEPS SHOULD THIS CALCULATION BE? Use you heating and cooling curve to help you understand the steps!

ANSWER (2 steps) p p Step 1 = raise temp of water from 55. 0 °C to 100. 0 °C Step 2 = vaporize water using heat of vaporization How much heat energy is required to bring 135. 5 g of water at 55. 0 °C to its boiling point and then vaporize it? q = mcΔT q = (135. 5 g)(4. 184 J/g°C)(100. 0°C-55. 0°C) = 25511. 94 to (3 sig figs) = 25500 J q = mass x heat of vaporization q = (135. 5 g)(2260 J/g) = = 306230 to (3 sig figs) = 306000 J ***ADD UP THE 2 NUMBERS TO GET THE OVERALL HEAT REQUIRED FOR THIS PROCESS

25500 J + 306000 J 331500 J = 332000 J

Example 3 p How much heat energy is required to convert 15. 0 g of ice at -12. 5 °C to steam at 123. 0 °C? p THINK!!! HOW MANY STEPS SHOULD THIS CALCULATION BE? ?

How much heat energy is required to convert 15. 0 g of ice at -12. 5 °C to steam at 123. 0 °C? = 5 steps qice = (15. 0 g)(2. 09 J/g°C)(0. 0 --12. 5°C) = 392 J qfus = (15. 0 g)(334 J/g) = 5, 010 J qwater = (15. 0 g)(4. 184 J/g°C)(100. 0 -0. 0°C) = 6, 280 J qvap = (15. 0 g)(2260 J/g) = 33, 900 J qsteam = (15. 0 g)(2. 03 J/g°C)(123. 0 -100. 0°C) = 700. J 46, 282 J 46, 300 J

Pop Quiz - How much heat energy is released to convert 25. 0 g of steam at 135. 0°C to ice at -15. 0°C?
 Chapter 7 heat transfer and change of phase
Chapter 7 heat transfer and change of phase Molar enthalpy units
Molar enthalpy units Entalpy
Entalpy Enthalpy calculation
Enthalpy calculation Determine enthalpy of reaction
Determine enthalpy of reaction Calculating average rate of change
Calculating average rate of change Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Tswett pronunciation
Tswett pronunciation Mobile phase and stationary phase
Mobile phase and stationary phase Stationary and mobile phase
Stationary and mobile phase Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Difference between phase voltage and line voltage
Difference between phase voltage and line voltage Adsorption chromatography
Adsorption chromatography