CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING








- Slides: 13

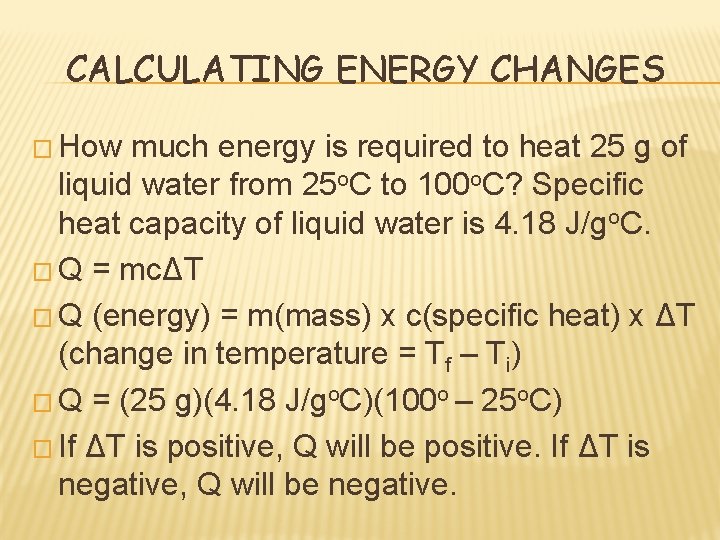
CALCULATING ENERGY CHANGES

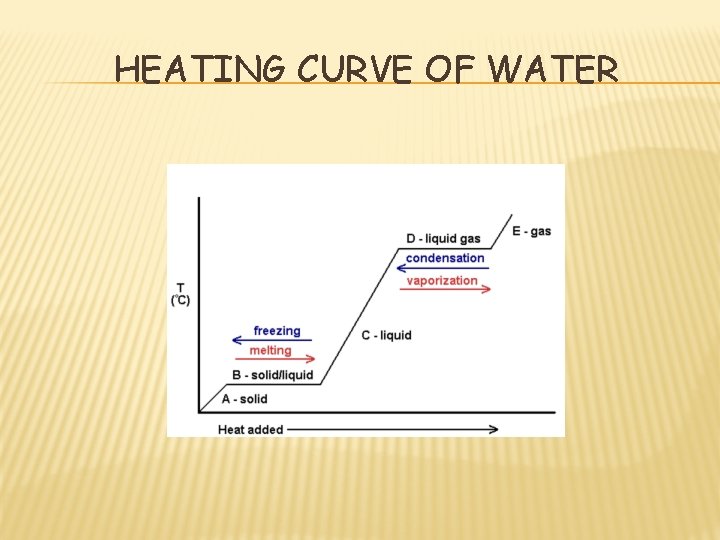
HEATING CURVE OF WATER

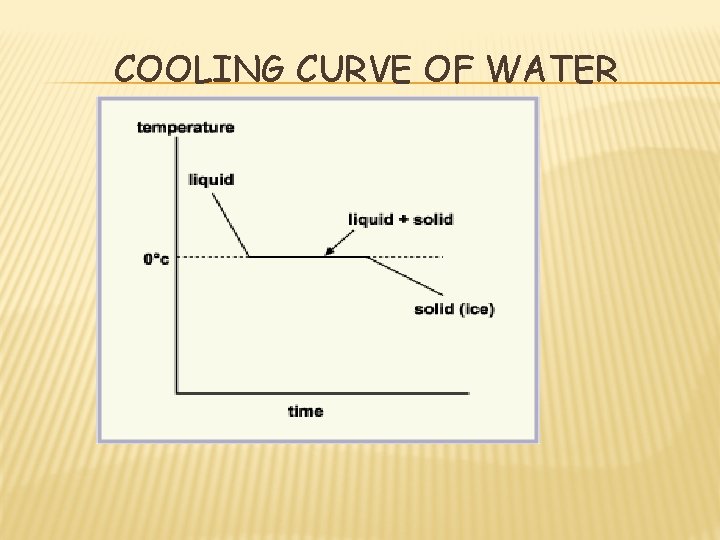
COOLING CURVE OF WATER

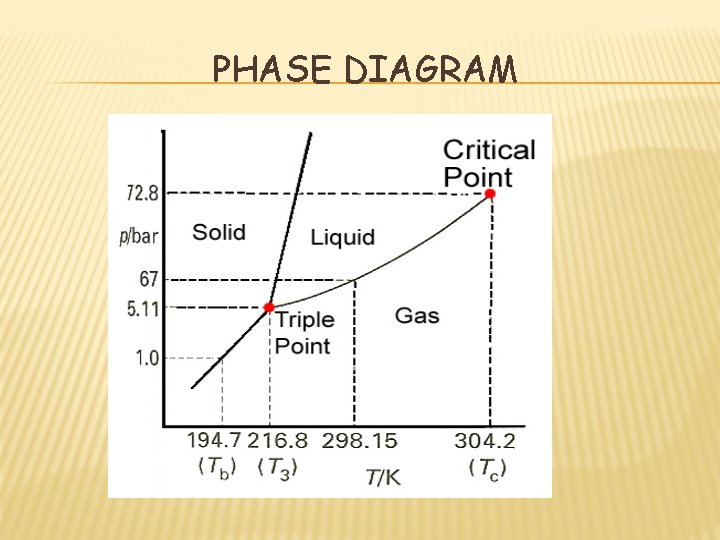
PHASE DIAGRAM

ENERGY CONVERSIONS � Energy (heat) may be expressed in joules or calories. � 1 calorie (cal) = 4. 184 joules (J) � How many joules in 60. 1 calories? � How many calories in 28. 4 J?

CALCULATING ENERGY CHANGES � How much energy is required to heat 25 g of liquid water from 25 o. C to 100 o. C? Specific heat capacity of liquid water is 4. 18 J/go. C. � Q = mcΔT � Q (energy) = m(mass) x c(specific heat) x ΔT (change in temperature = Tf – Ti) � Q = (25 g)(4. 18 J/go. C)(100 o – 25 o. C) � If ΔT is positive, Q will be positive. If ΔT is negative, Q will be negative.

� How many joules of heat are given off when 5. 0 g of water cools from 75 o. C to 25 oc? (Specific heat of water = 4. 18 J/go. C)

YOUR ASSIGNMENT: DUE MON. 3/29 � Page 329 Practice Problem 10. 2 � Page 330 Practice Problem 10. 3 � Page 333 Section 10. 2 Review Questions 2 -6

ENERGY DURING PHASE CHANGE � Energy is increasing/decreasing during phase changes even though temp. remains constant. � Q = mass(m) x heat of fusion (Hf) for melting and freezing � Q = mass(m) x heat of vaporization (Hv) for boiling and condensing � Values will be positive for melting and boiling � Values will be negative for freezing and condensing

� How many calories are given off when 85 g of steam condense to liquid water? (Hv= 539. 4 cal/g)

� How many joules does it take to melt 35 g of ice at 0 o. C? (Hf = 333 J/g)

� How many joules are required to convert 10. 0 g of ice at -10. 0 o. C to steam at 150. o. C? � Given: Specific heat of � Specific heat of water = 4. 184 J/g C° � Specific heat of Steam = 2. 03 J/g C° � Specific heat of ice = 2. 06 J/g C° � Hv water = 539. 4 cal/g � Hf water = 333. 0 J/g � 5 part calculation

YOUR ASSIGNMENT: DUE TUES. 3/30 � Page 497 Practice Problem 14. 2 � Page 497 Section 14. 1 Review 1, 3, 4 -7 � Page 503 Section 14. 2 Review 1, 4, 6