Calculating and using oxidation numbers 1 The oxidation

Calculating and using oxidation numbers

1 The oxidation number of any free, uncombined element is zero. This includes polyatomic molecules of elements such as H 2, O 3 and S 8. 2 The charge on a simple (monatomic) ion is the oxidation number of the element in that ion. In a polyatomic ion, the sum of the oxidation numbers of the atoms in that ion is equal to the charge on the ion. 3 In compounds (whether ionic or covalent), the sum of the oxidation numbers of all atoms in the compound is zero. 4 The oxidation number of oxygen is – 2, except in the case of peroxides where it is – 1. 5 The oxidation number of hydrogen is +1, except in the case of metallic hydrides where it is – 1.
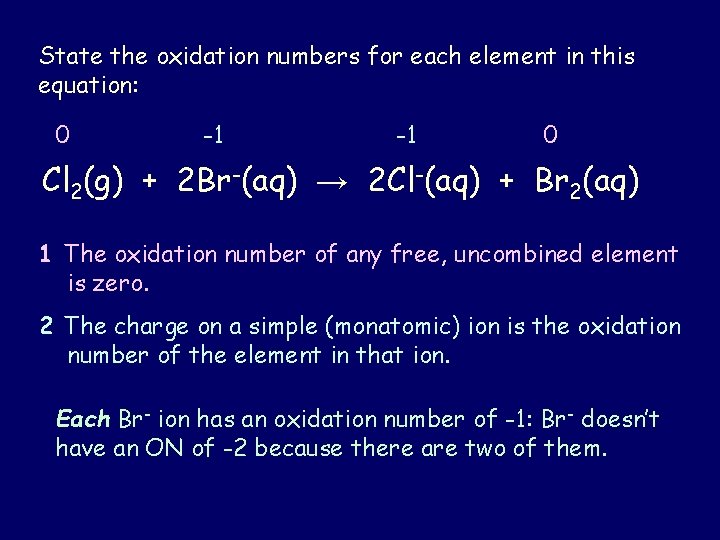
State the oxidation numbers for each element in this equation: 0 -1 -1 0 Cl 2(g) + 2 Br-(aq) → 2 Cl-(aq) + Br 2(aq) 1 The oxidation number of any free, uncombined element is zero. 2 The charge on a simple (monatomic) ion is the oxidation number of the element in that ion. Each Br- ion has an oxidation number of -1: Br- doesn’t have an ON of -2 because there are two of them.

State the oxidation numbers for each element in this equation: -2 -2 2 Mn. O 4 - + 3 H 2 O 2 + 2 H+ → 2 Mn. O 2 + 3 O 2 + 4 H 2 O Hydrogen peroxide 1 The oxidation number of any free, uncombined element is 0 0. +1 5 The oxidation number of hydrogen is +1, -2 except… 4 The oxidation number of oxygen is – 2 in the case of peroxides where it is -1 – 1. 2 In a polyatomic ion, the sum of the oxidation numbers of the atoms in that ion is equal to the charge on the ion. Mn. O 4 -: Mn + 4(-2) = -1 Mn - 8 = -1 Mn = +7 3 In compounds (whether ionic or covalent), the sum of the oxidation numbers of all atoms in the compound is zero. Mn. O 2: Mn + 2(-2) = 0 Mn - 4 =0 Mn = +4
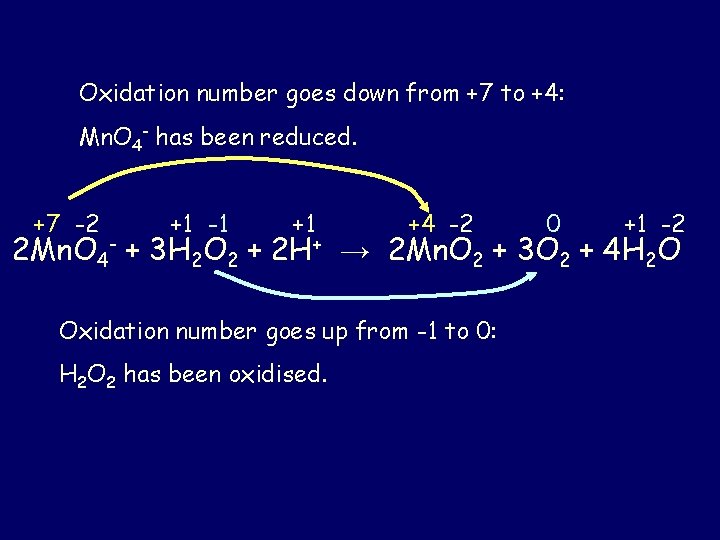
Oxidation number goes down from +7 to +4: Mn. O 4 - has been reduced. +7 -2 +1 -1 +1 +4 -2 0 +1 -2 2 Mn. O 4 - + 3 H 2 O 2 + 2 H+ → 2 Mn. O 2 + 3 O 2 + 4 H 2 O Oxidation number goes up from -1 to 0: H 2 O 2 has been oxidised.
- Slides: 5