Calculate the limiting reactant the percent excess reactant














- Slides: 33



ตวอยาง Calculate the limiting reactant. the percent excess reactant. the degree of completion. kg of CO produced. 3

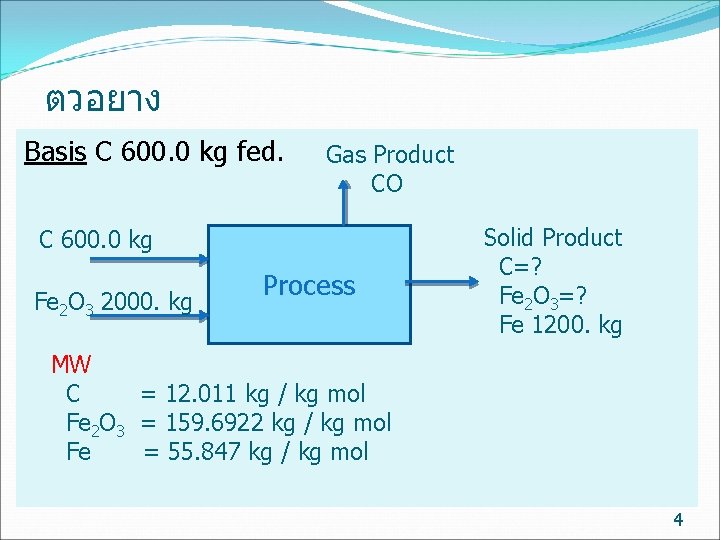
ตวอยาง Basis C 600. 0 kg fed. Gas Product CO C 600. 0 kg Fe 2 O 3 2000. kg Process Solid Product C=? Fe 2 O 3=? Fe 1200. kg MW C = 12. 011 kg / kg mol Fe 2 O 3 = 159. 6922 kg / kg mol Fe = 55. 847 kg / kg mol 4

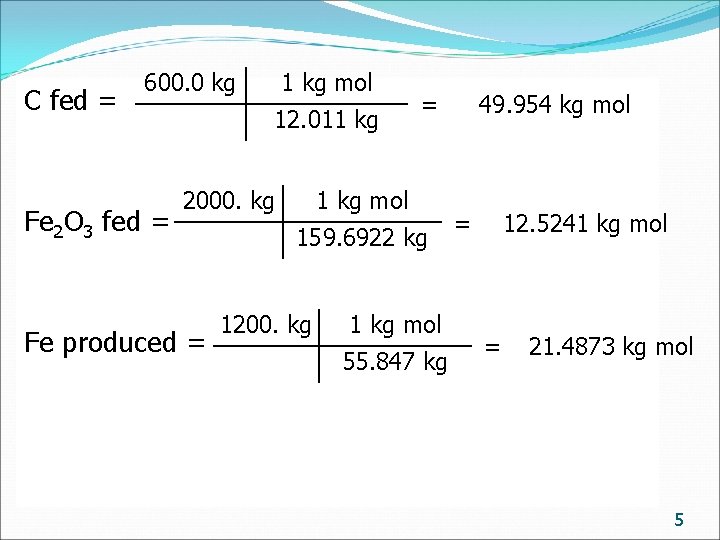
C fed = 600. 0 kg Fe 2 O 3 fed = 1 kg mol 12. 011 kg 2000. kg Fe produced = = 1 kg mol 159. 6922 kg 1200. kg 1 kg mol 55. 847 kg 49. 954 kg mol = 12. 5241 kg mol = 21. 4873 kg mol 5


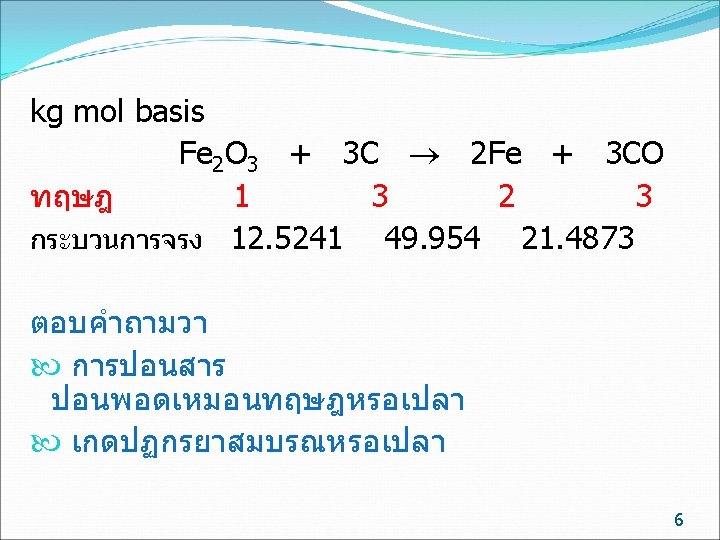
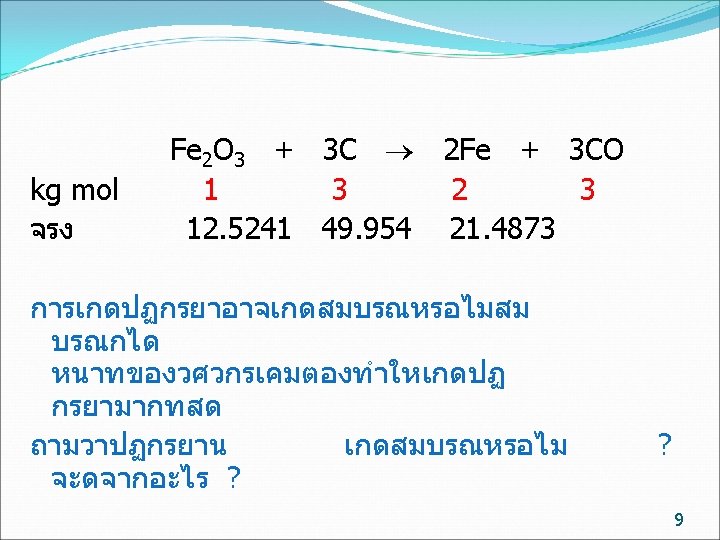
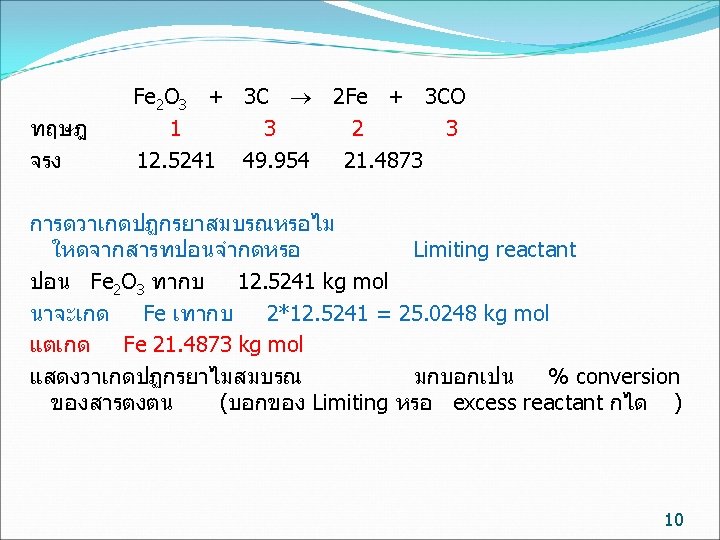
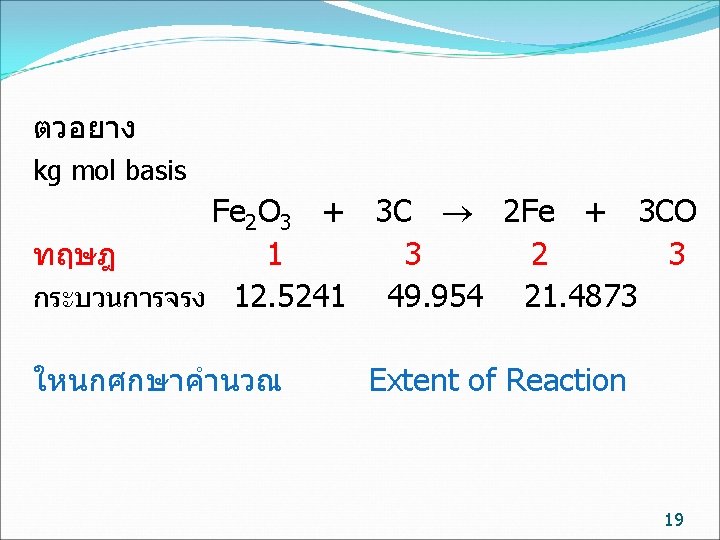
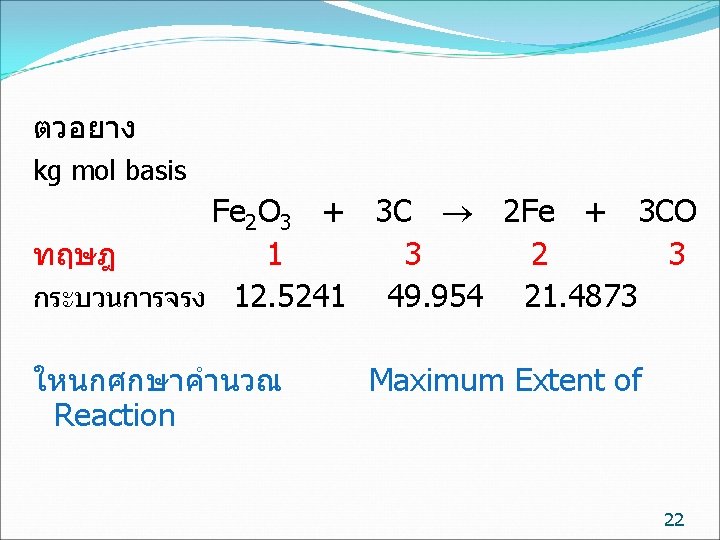
kg mol basis ทฤษฎ จรง Fe 2 O 3 + 3 C 2 Fe + 3 CO 1 3 2 3 12. 5241 49. 954 21. 4873 การปอนสาร ไมจำเปนตองปอนพอด จะมตวหนงปอนนอย (Limiting reactant) อกตวปอนมากเกนไป (Excess reactant) ถามวาสารตวใดเปน Limiting reactant Fe 2 O 3 /C C / Fe 2 O 3 ทฤษฎ 1/3= 0. 333 3/1= 3 ปอนจรง 12. 5241/49. 954 = 0. 251 49. 954 /12. 5241/ =3. 989 Fe 2 O 3 เปน Limiting reactant C เปน Excess reactant 7




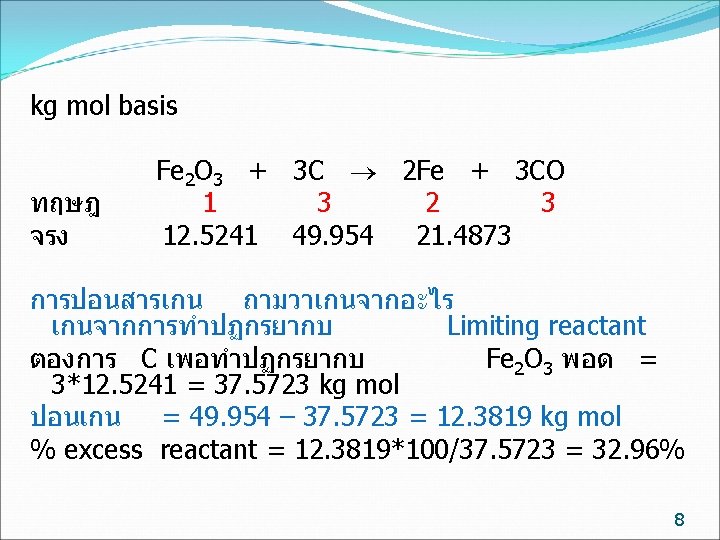
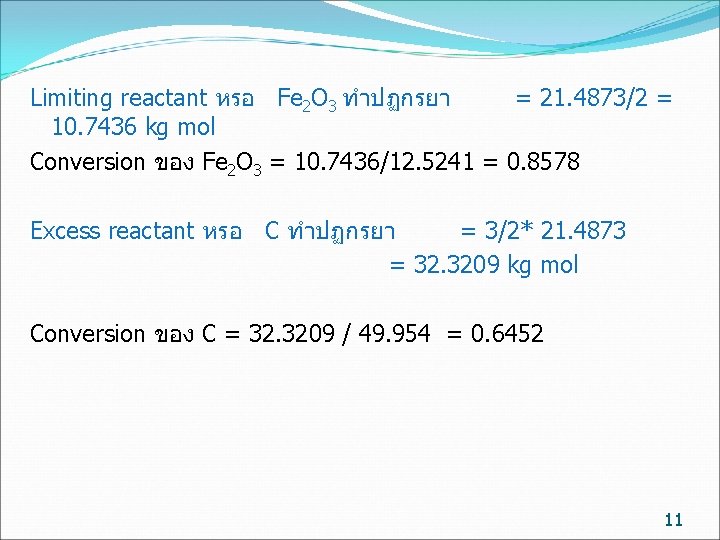
Limiting reactant หรอ Fe 2 O 3 ทำปฏกรยา = 21. 4873/2 = 10. 7436 kg mol Conversion ของ Fe 2 O 3 = 10. 7436/12. 5241 = 0. 8578 Excess reactant หรอ C ทำปฏกรยา = 3/2* 21. 4873 = 32. 3209 kg mol Conversion ของ C = 32. 3209 / 49. 954 = 0. 6452 11


Quiz # 4 Stochiometry 13



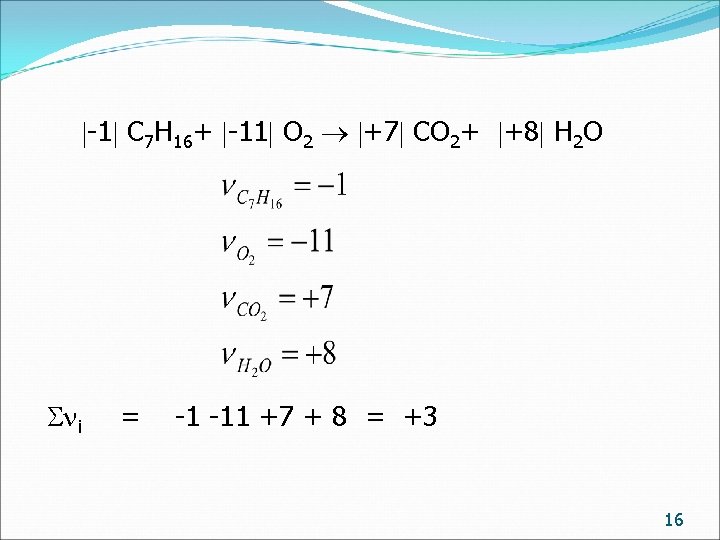
-1 C 7 H 16+ -11 O 2 +7 CO 2+ +8 H 2 O i = -1 -11 +7 + 8 = +3 16


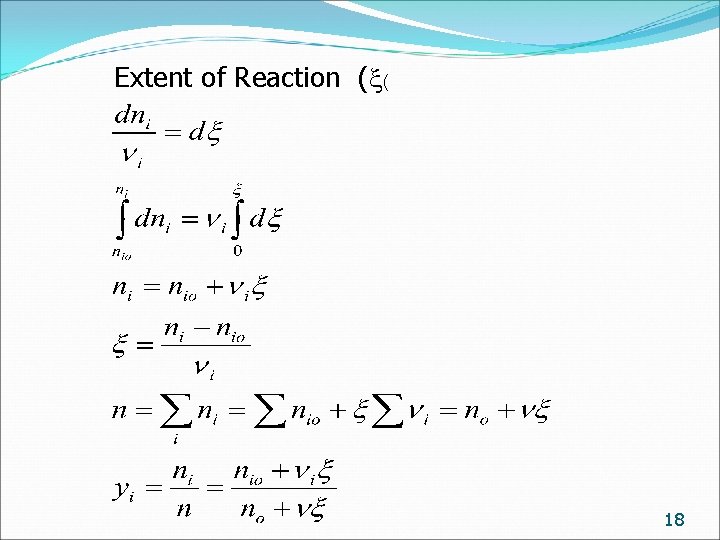
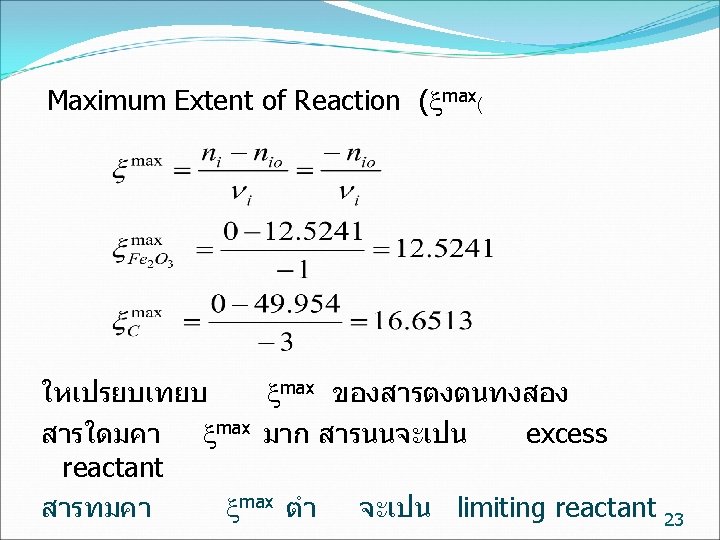
Extent of Reaction ( ( 18


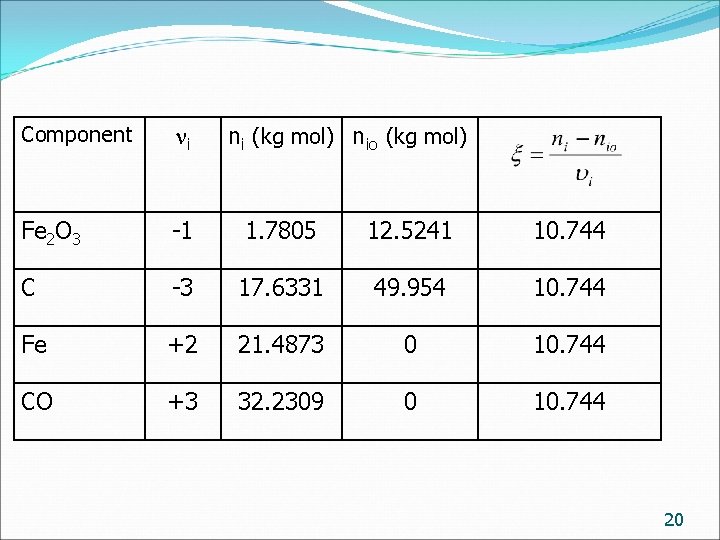
Component i Fe 2 O 3 -1 1. 7805 12. 5241 10. 744 C -3 17. 6331 49. 954 10. 744 Fe +2 21. 4873 0 10. 744 CO +3 32. 2309 0 10. 744 ni (kg mol) nio (kg mol) 20


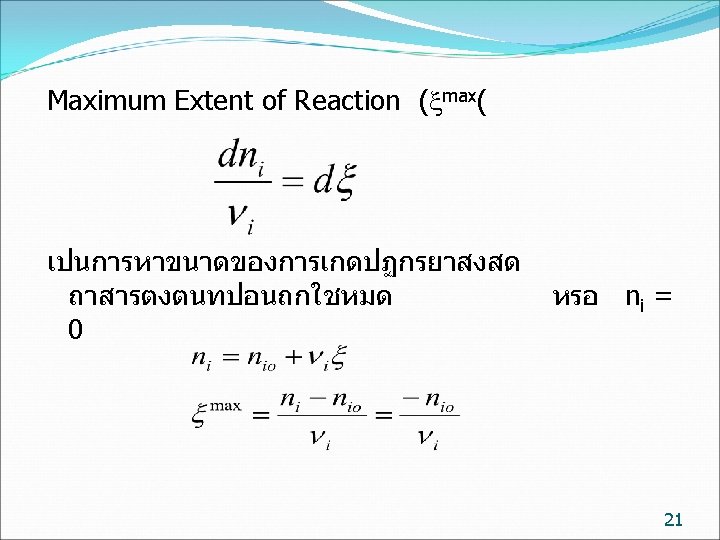
ตวอยาง kg mol basis Fe 2 O 3 + 3 C 2 Fe + 3 CO ทฤษฎ 1 3 2 3 กระบวนการจรง 12. 5241 49. 954 21. 4873 ใหนกศกษาคำนวณ Reaction Maximum Extent of 22



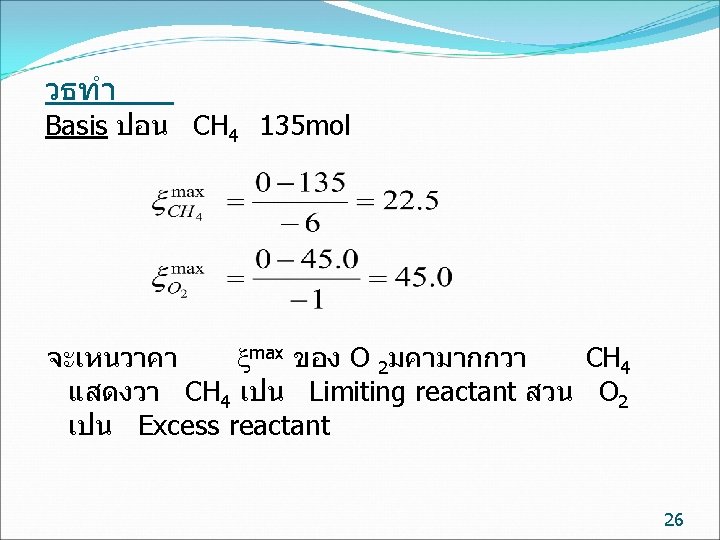
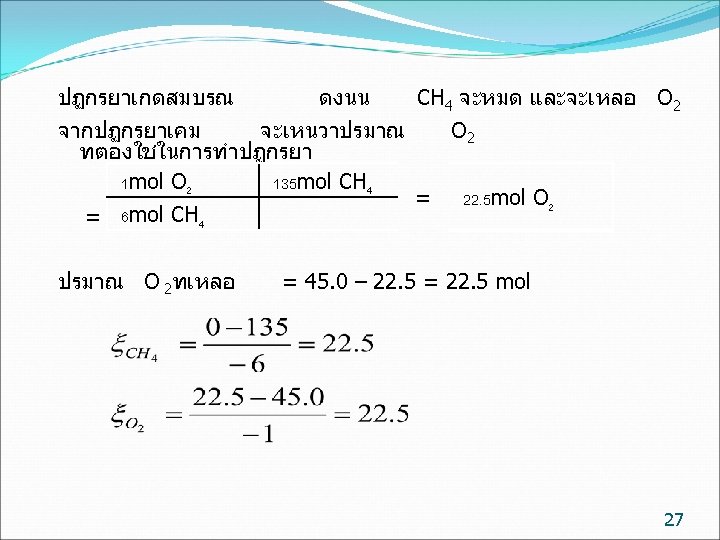
ตวอยาง (9. 18( In the reaction in which 135 moles of methane and 45. 0 moles of oxygen are fed into a reactor, if the reaction goes to completion, calculate the extent of reaction. 6 CH 4 + O 2 2 C 2 H 2 + 2 CO +10 H 2 25



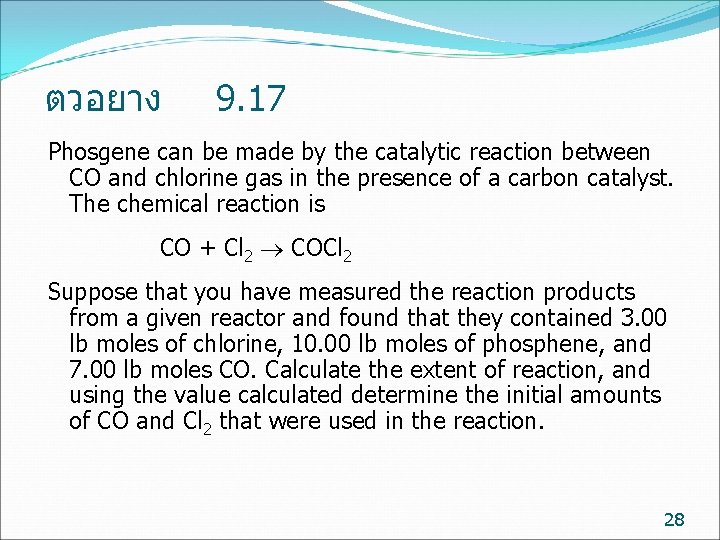
ตวอยาง 9. 17 Phosgene can be made by the catalytic reaction between CO and chlorine gas in the presence of a carbon catalyst. The chemical reaction is CO + Cl 2 COCl 2 Suppose that you have measured the reaction products from a given reactor and found that they contained 3. 00 lb moles of chlorine, 10. 00 lb moles of phosphene, and 7. 00 lb moles CO. Calculate the extent of reaction, and using the value calculated determine the initial amounts of CO and Cl 2 that were used in the reaction. 28

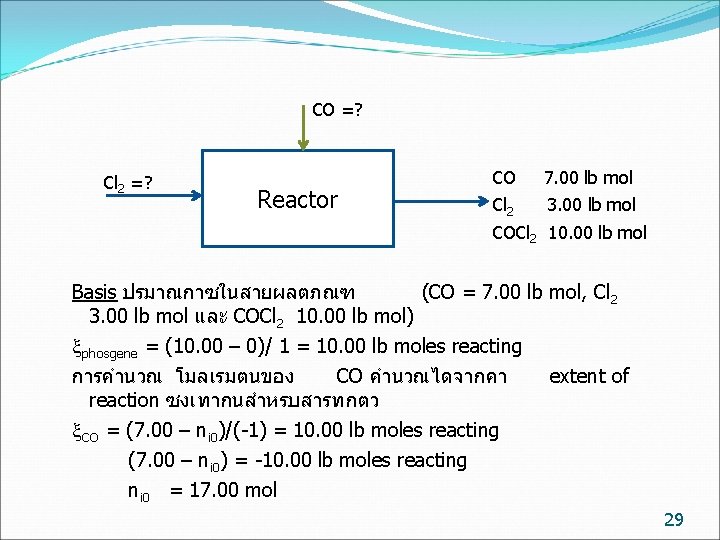
CO =? Cl 2 =? Reactor CO 7. 00 lb mol Cl 2 3. 00 lb mol COCl 2 10. 00 lb mol Basis ปรมาณกาซในสายผลตภณฑ (CO = 7. 00 lb mol, Cl 2 3. 00 lb mol และ COCl 2 10. 00 lb mol) phosgene = (10. 00 – 0)/ 1 = 10. 00 lb moles reacting การคำนวณ โมลเรมตนของ CO คำนวณไดจากคา reaction ซงเทากนสำหรบสารทกตว CO = (7. 00 – ni 0)/(-1) = 10. 00 lb moles reacting extent of (7. 00 – ni 0) = -10. 00 lb moles reacting ni 0 = 17. 00 mol 29


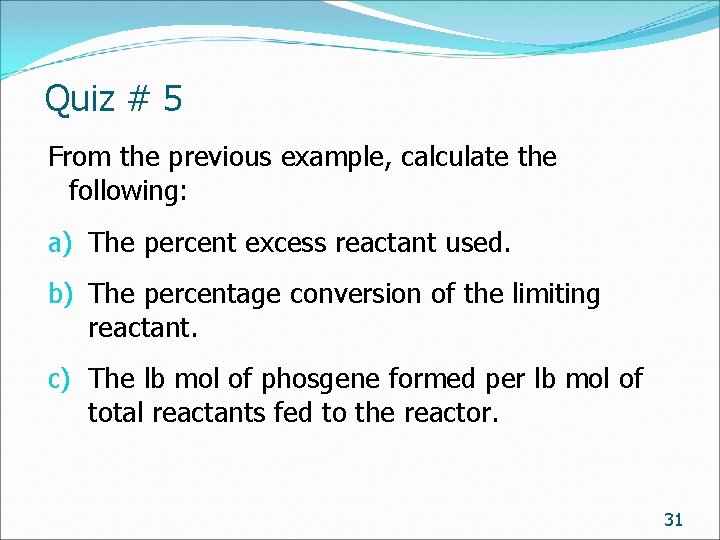
Quiz # 5 From the previous example, calculate the following: a) The percent excess reactant used. b) The percentage conversion of the limiting reactant. c) The lb mol of phosgene formed per lb mol of total reactants fed to the reactor. 31

Quiz # 5 9. 23 page 256 32

Homework 9. 27 9. 28 9. 30 33