Calcium chloride Ca Cl is a pure substance

Calcium chloride, Ca. Cl, is a pure substance used on roads to control dust and to melt ice and snow. What type of matter is this material? A Element B Atom C Compound D Gas
Elements join together to become compounds because of their valence electrons
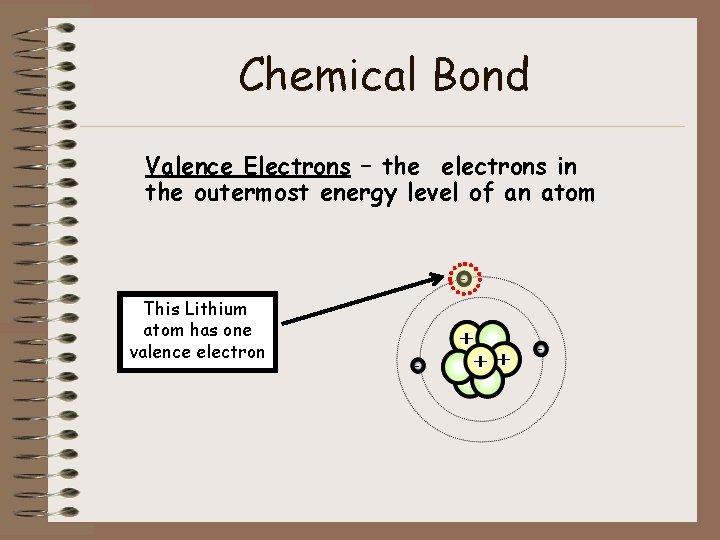
Chemical Bond Valence Electrons – the electrons in the outermost energy level of an atom This Lithium atom has one valence electron
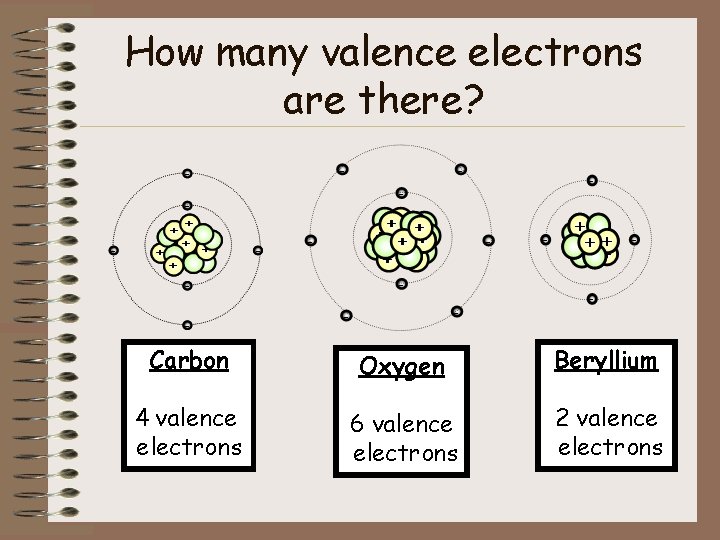
How many valence electrons are there? Carbon Oxygen Beryllium 4 valence electrons 6 valence electrons 2 valence electrons
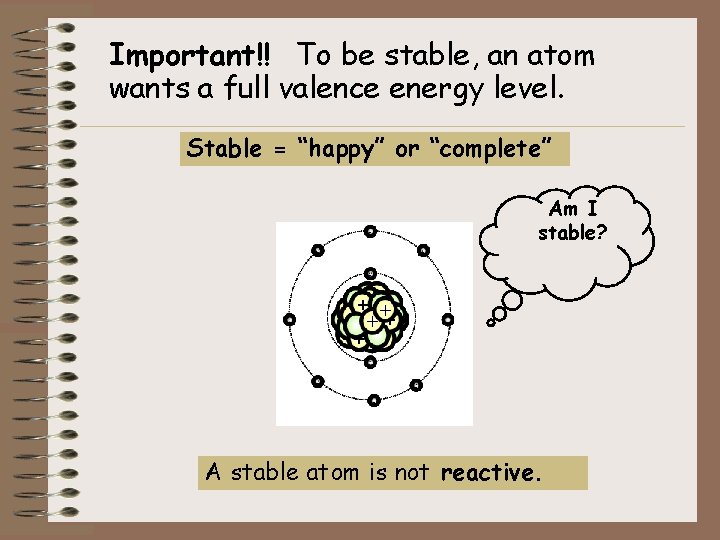
Important!! To be stable, an atom wants a full valence energy level. Stable = “happy” or “complete” Am I stable? A stable atom is not reactive.

Look through your Bohr models and find those atoms that are already stable. This atom is stable because…
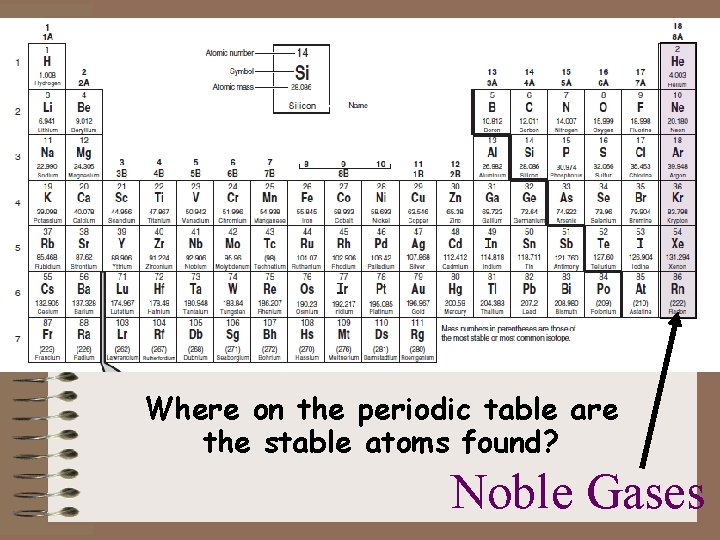
Where on the periodic table are the stable atoms found? Noble Gases
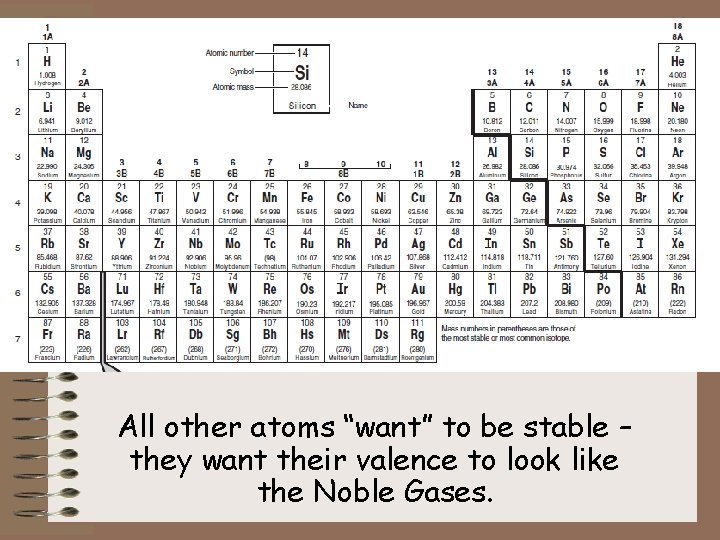
All other atoms “want” to be stable – they want their valence to look like the Noble Gases.

How close are the other atoms to looking like a noble gas? Try it! Sort your cards by the number of valence electrons the atom has 1 2 3 4 5 6 7
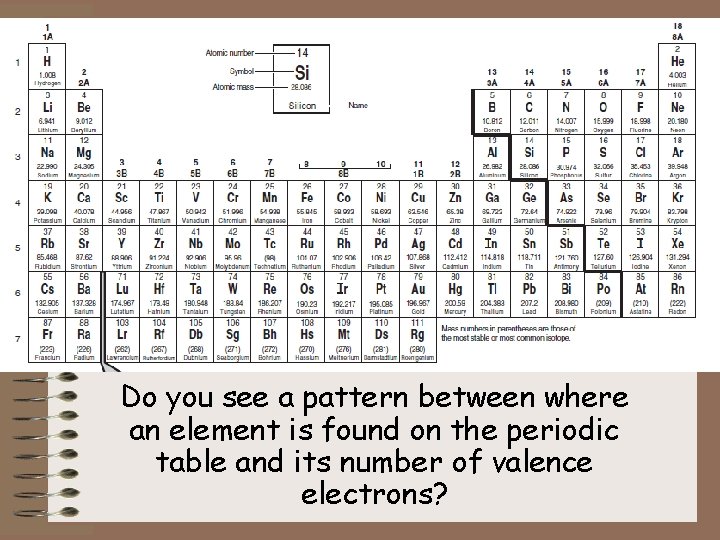
Do you see a pattern between where an element is found on the periodic table and its number of valence electrons?
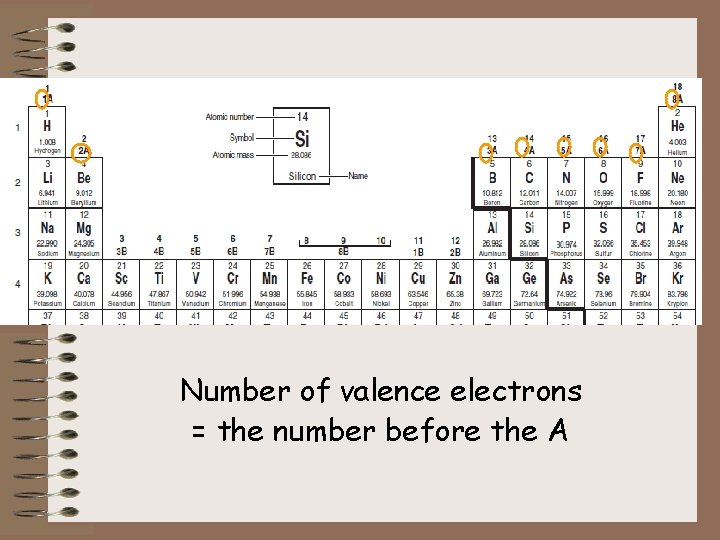
Number of valence electrons = the number before the A

Reactivity If an atom is not stable, it will want to get more electrons or give some electrons away to become like a nearby noble gas. This is called Reactivity

Comparing Reactivity Put these cards in a row: C N O F Ne Which of these is the least reactive? (Hint: which is stable? ) Which of these do you think is the most reactive?
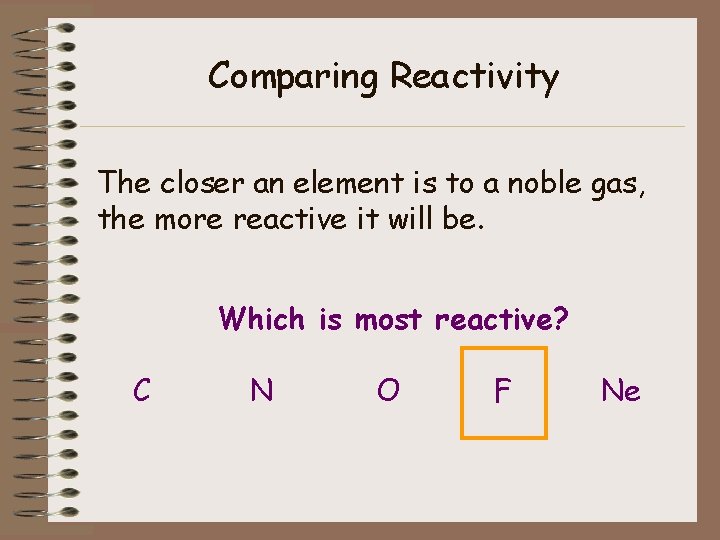
Comparing Reactivity The closer an element is to a noble gas, the more reactive it will be. Which is most reactive? C N O F Ne
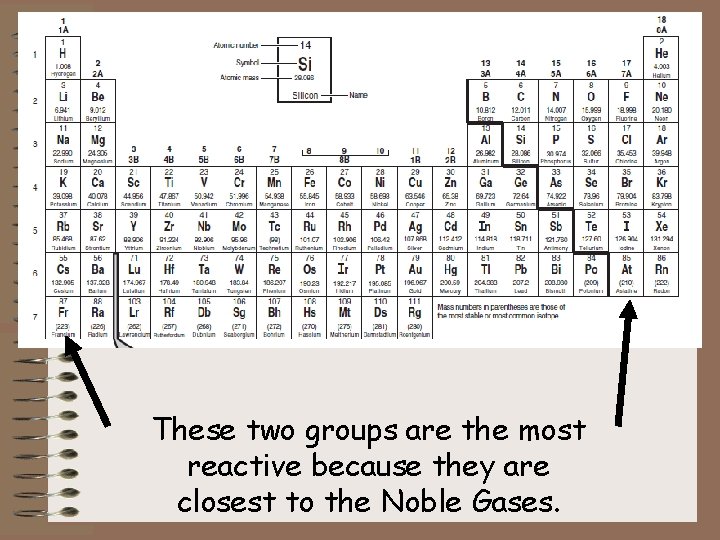
These two groups are the most reactive because they are closest to the Noble Gases.

Practice
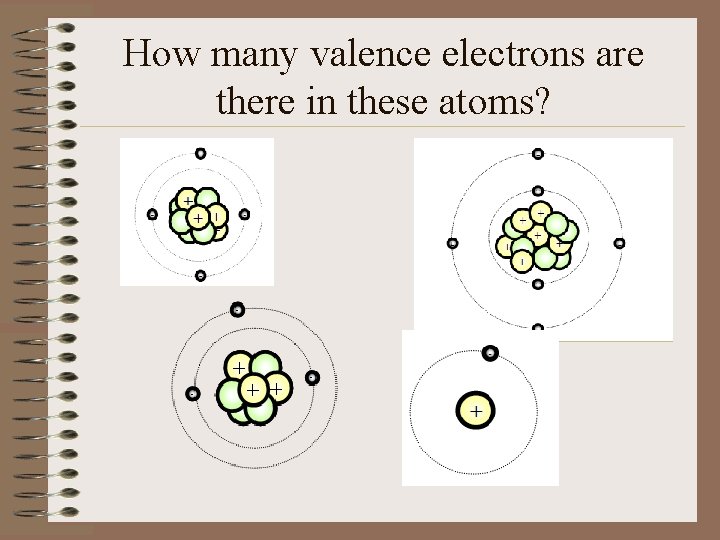
How many valence electrons are there in these atoms?
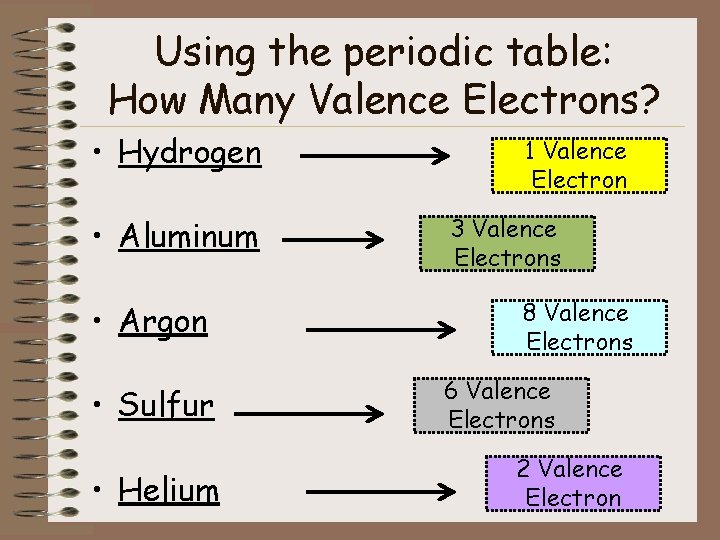
Using the periodic table: How Many Valence Electrons? • Hydrogen • Aluminum • Argon • Sulfur • Helium 1 Valence Electron 3 Valence Electrons 8 Valence Electrons 6 Valence Electrons 2 Valence Electron
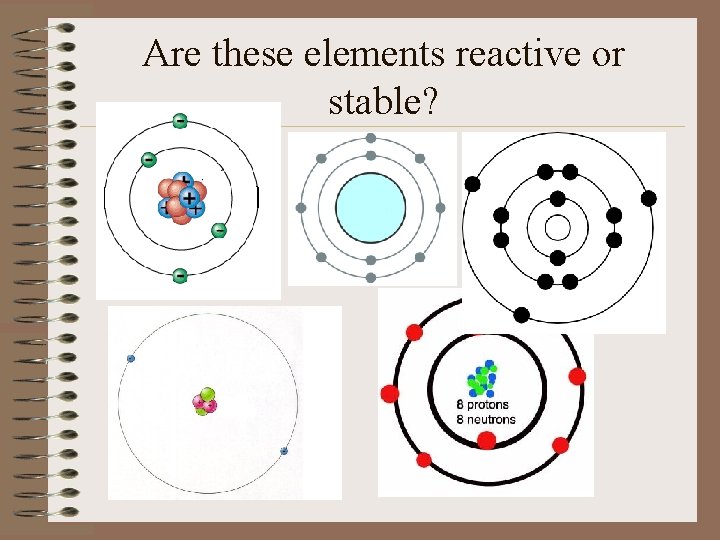
Are these elements reactive or stable?

Which element is the most reactive… Aluminum or Chlorine?

Which element is the most reactive… Sulfur or Phosphorus?

Which element is the most reactive… Nitrogen or Neon?

Which element is the most reactive… Sulfur, Chlorine or Argon?

Which element is the most reactive… Nitrogen, Oxygen or Fluorine?
- Slides: 24