C4 Atomic Structure The nucleus and the moving





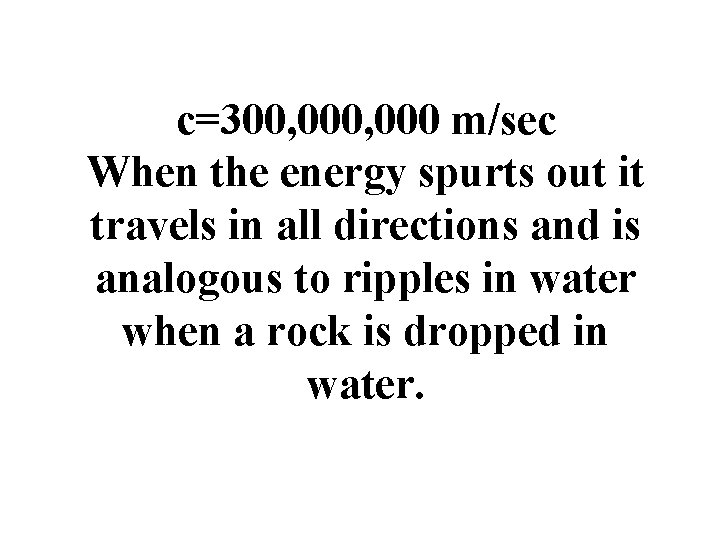












- Slides: 23

C-4 Atomic Structure The nucleus and the moving electrons.

Law of Definite Proportions • Specific substances always contain the same elements in the same ratio by mass. • Ex. Water is made up of about 2 grams of hydrogen to 16 grams of oxygen. Therefore we come up with the formula for water to be H 2 O.

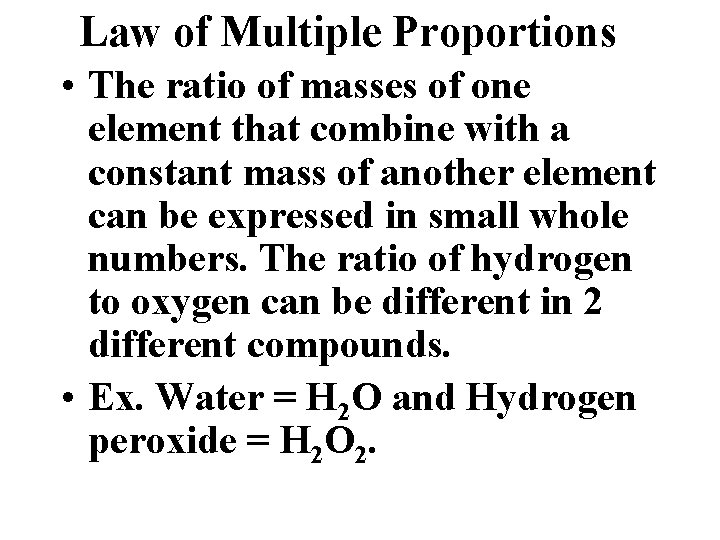
Law of Multiple Proportions • The ratio of masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. The ratio of hydrogen to oxygen can be different in 2 different compounds. • Ex. Water = H 2 O and Hydrogen peroxide = H 2 O 2.

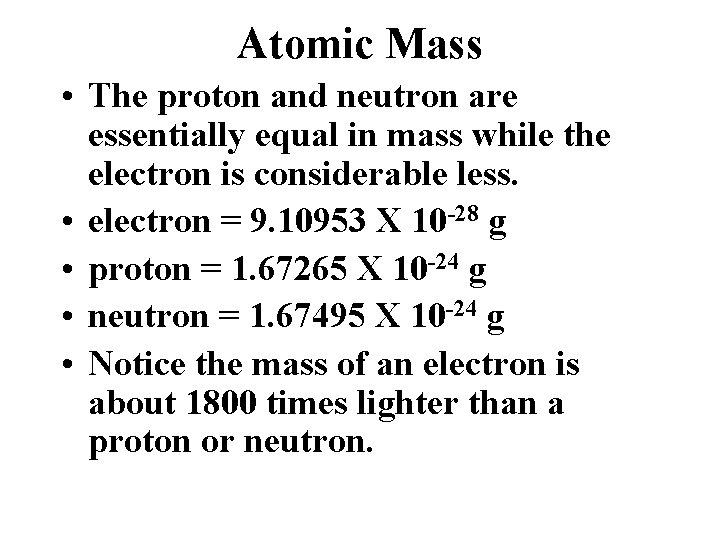
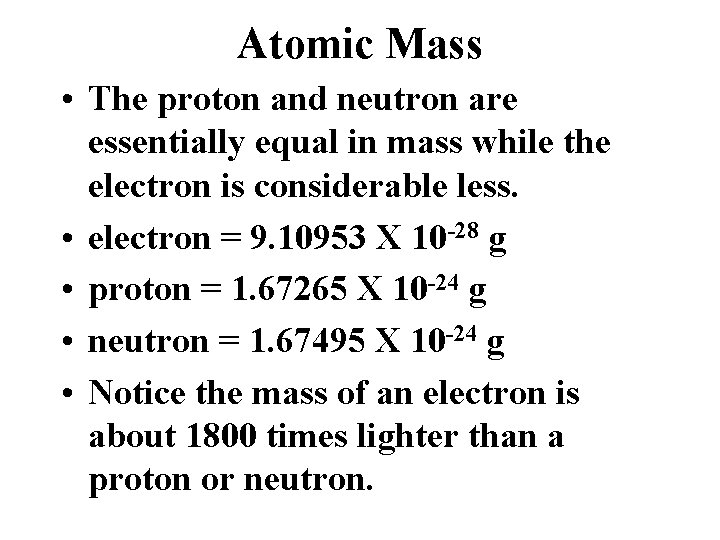
Atomic Mass • The proton and neutron are essentially equal in mass while the electron is considerable less. • electron = 9. 10953 X 10 -28 g • proton = 1. 67265 X 10 -24 g • neutron = 1. 67495 X 10 -24 g • Notice the mass of an electron is about 1800 times lighter than a proton or neutron.

• The orbiting of electrons around the nucleus is much like a satellite orbiting the earth. • Electrons are continuously giving off energy thus should slow down and be pulled in by the nucleus and the + charge: Just as a satellite would be pulled into the earth if it lost energy and slowed down.

• Electrons never collapse into the nucleus even though they are continuously giving off energy.

Electromagnetic Radiation The energy given off by electrons is called electromagnetic radiation. • This energy is not coming off continuously but in spurts. • Once the energy comes off it travels at the speed of light. (c) •
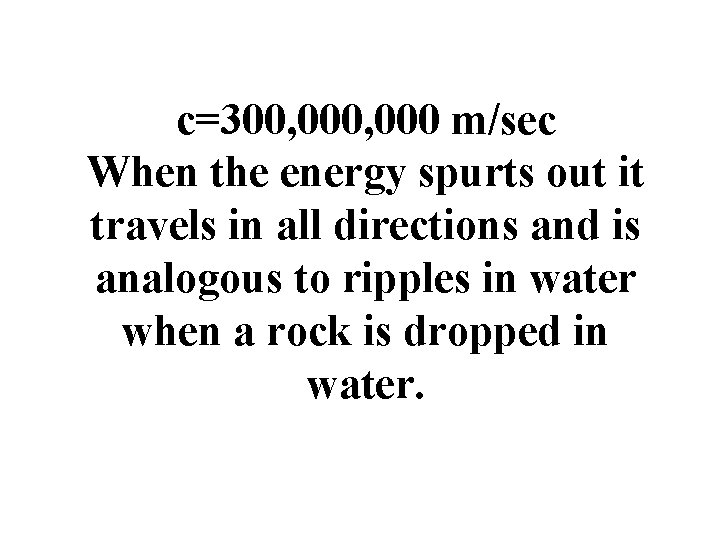
c=300, 000 m/sec When the energy spurts out it travels in all directions and is analogous to ripples in water when a rock is dropped in water.

• Therefore we can say that electromagnetic radiation (energy) comes off in waves. • The longer the time between emissions of EMR from electrons the further the waves are apart. The distance between 2 successive waves is called the energy’s wavelength.

• The more frequently an electron emits energy, the higher the number of waves that come off each second. The # of waves emitted per second is called frequency.

• The larger the frequency of emission of energy, the closer the waves are to each other or in other words the smaller the wavelength. • The product of the wavelength and frequency is always a constant and that constant has been found to be the speed of light. “ wavelength X frequency = c” • lambda X f = c

• The greater the frequency of energy emission, the more energy you’ll be bombarded with each second. Therefore, the frequency determines the amount of energy coming off an electron. • Planck was the first to determine the equation for energy and frequency. • E = h x f ( h is a constant)

Photons • Let’s take a closer look at these waves of energy that are being emitted by electrons. • The energy coming off is in packets or quanta so that waves would look like:

• These little packets of energy are called photons. • These photons eventually hit some form of matter and are absorbed thus giving the matter more energy.

• This energy is absorbed on the atomic level of the matter (in the electrons) and thus these atoms have more energy. • An atom which has absorbed energy in this way is called an excited atom. • When excited atoms radiate energy, the radiation must be given off in photons.

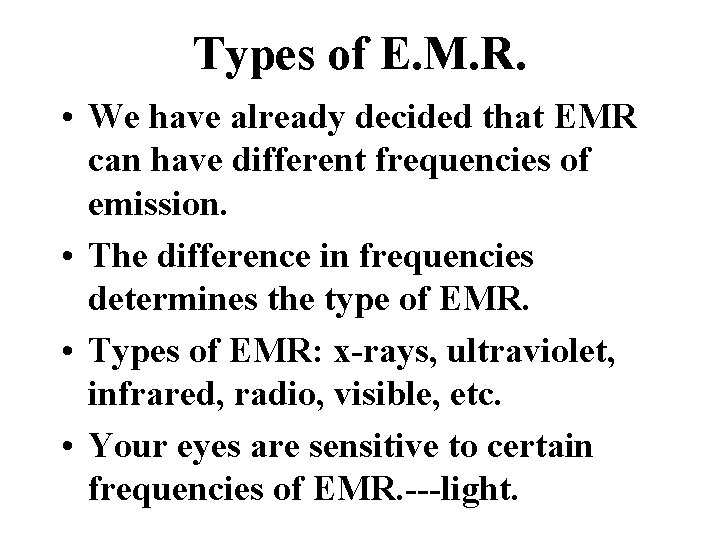
Types of E. M. R. • We have already decided that EMR can have different frequencies of emission. • The difference in frequencies determines the type of EMR. • Types of EMR: x-rays, ultraviolet, infrared, radio, visible, etc. • Your eyes are sensitive to certain frequencies of EMR. ---light.

Spectra of Atoms • Different elements are excited differently when energy is added and thus emit energy differently also. • This fact can be used to identify the elements. • Know the spectrum p. 96

Radiation • There are 3 forms of radiation with the first 2 being comprised of particles. – 1. alpha particle-is a helium nucleus made up of 2 protons and 2 neutrons. – 2. beta particle-is a high speed electron from a radioactive nuclei. – 3. gamma rays-very high energy x-rays

The Hydrogen Atom and the Quantum Theory • The electrons travel in many different orbits around the nucleus. The smallest of these orbits is called the ground state. • The further out an electron travels the more energy it has.

• Therefore, an electron at ground state has the lowest amount of energy. • An electron needs to gain energy to move to an outer orbit, it gives off energy when it moves into a closer orbit.

Atomic Mass • The proton and neutron are essentially equal in mass while the electron is considerable less. • electron = 9. 10953 X 10 -28 g • proton = 1. 67265 X 10 -24 g • neutron = 1. 67495 X 10 -24 g • Notice the mass of an electron is about 1800 times lighter than a proton or neutron.

Average Atomic Mass • Not all atoms of the same element have the same mass. They have a mass that is close to an average for that element. • The average mass is calculated by taking the masses for all the isotopes of an element and their relative amount of existence.

• Ex. Hydrogen has an atomic mass of 1. 00797 amu that is based on 2 isotopes, deuterium-2 amu and protium -1 amu. • Read & Work the sample on p. 103