c S Coates 2012 Everything around us is












- Slides: 48

(c) S. Coates 2012

�Everything around us is made of atoms. These atoms are constantly interacting with each other. �We call those interactions chemical reactions, and they are happening EVERYWHERE. (c) S. Coates 2012

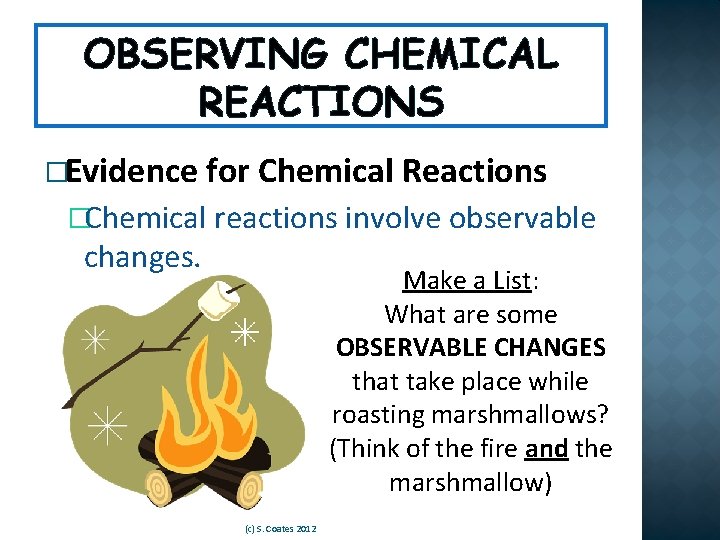
OBSERVING CHEMICAL REACTIONS �Evidence for Chemical Reactions �Chemical reactions involve observable changes. Make a List: What are some OBSERVABLE CHANGES that take place while roasting marshmallows? (Think of the fire and the marshmallow) (c) S. Coates 2012

OBSERVING CHEMICAL REACTIONS �Evidence for Chemical Reactions �The list you just came up with can be sorted into TWO lists… 1. Changes in Properties 2. Changes in Energy These are the TWO Main kinds of Observable Changes that take place in a chemical reaction. (c) S. Coates 2012

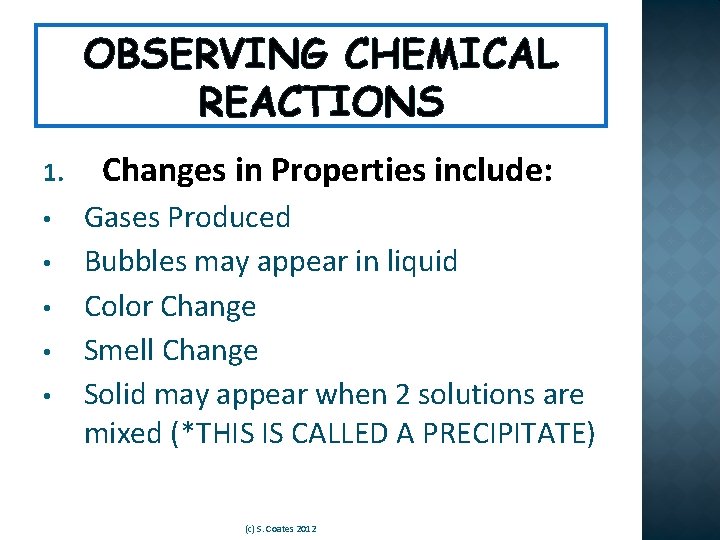
OBSERVING CHEMICAL REACTIONS 1. • • • Changes in Properties include: Gases Produced Bubbles may appear in liquid Color Change Smell Change Solid may appear when 2 solutions are mixed (*THIS IS CALLED A PRECIPITATE) (c) S. Coates 2012

�Watch the video below and look for CHANGES IN PROPERTIES. (c) S. Coates 2012

1. OBSERVING CHEMICAL REACTIONS Changes in Properties �Q: What change in property did the Diet Coke go through during the chemical reaction in the video? �A: Increased “fizziness” or CO 2 was the most observable change. (c) S. Coates 2012

�For example, sometimes TWO liquids will mix together and will form a SOLID. We call this solid a PRECIPITATE. (c) S. Coates 2012

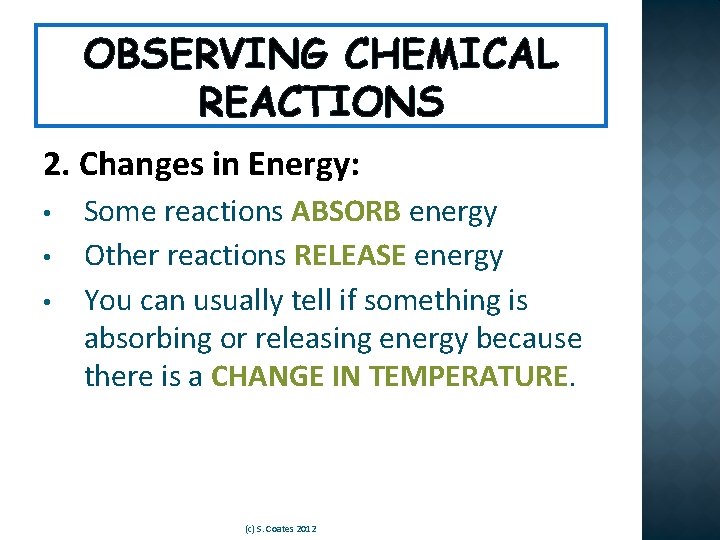
OBSERVING CHEMICAL REACTIONS 2. Changes in Energy: • • • Some reactions ABSORB energy Other reactions RELEASE energy You can usually tell if something is absorbing or releasing energy because there is a CHANGE IN TEMPERATURE. (c) S. Coates 2012

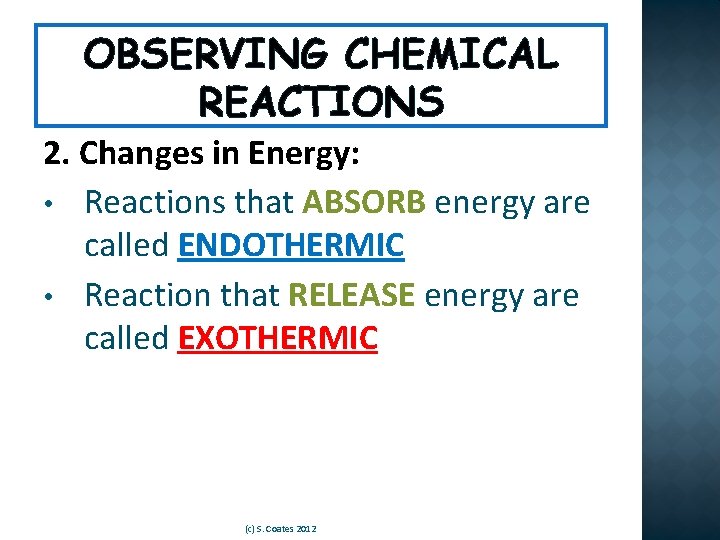
OBSERVING CHEMICAL REACTIONS 2. Changes in Energy: • Reactions that ABSORB energy are called ENDOTHERMIC • Reaction that RELEASE energy are called EXOTHERMIC (c) S. Coates 2012

OBSERVING CHEMICAL REACTIONS �The BIGGEST observable characteristic of a chemical reaction is the production of NEW MATERIALS �The NEW MATERIALS will have properties that are different from those of the starting materials. (c) S. Coates 2012

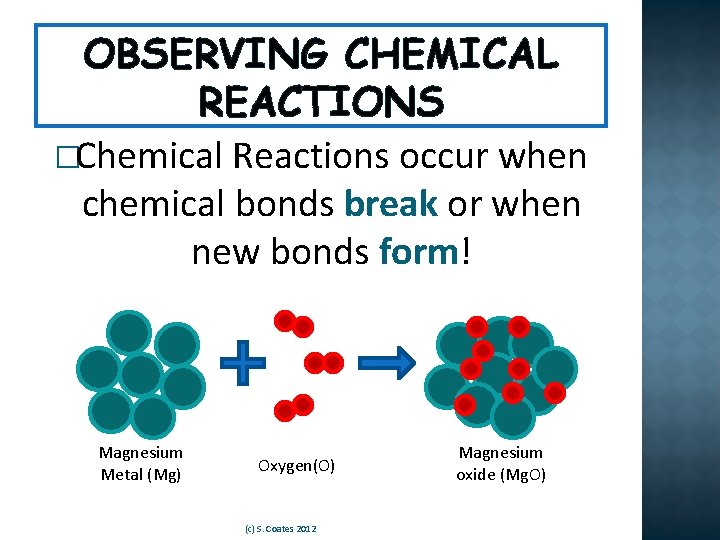
OBSERVING CHEMICAL REACTIONS �Chemical Reactions occur when chemical bonds break or when new bonds form! Magnesium Metal (Mg) Oxygen(O) (c) S. Coates 2012 Magnesium oxide (Mg. O)

OBSERVING CHEMICAL REACTIONS 2. Changes in Energy �Chemical Reactions happen because of the way chemicals are stuck together or “bonded”. �Some bonds are strong and hard to change…think of Glass. �Some bonds are weak…think of Wood. (c) S. Coates 2012

OBSERVING CHEMICAL REACTIONS �STOP 1. 2. 3. 4. AND REVIEW What are some examples of evidence for a chemical reaction? What two kinds of energy changes can take place during a chemical reaction? What happens to the chemical bonds in a substance during a chemical reaction? When a solid forms as two solutions are mixed together, what is that solid called? (c) S. Coates 2012

(c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS �Group Activity! �Materials needed per group: 20 different coins, each of any value Paper and Pencil to record results (c) S. Coates 2012

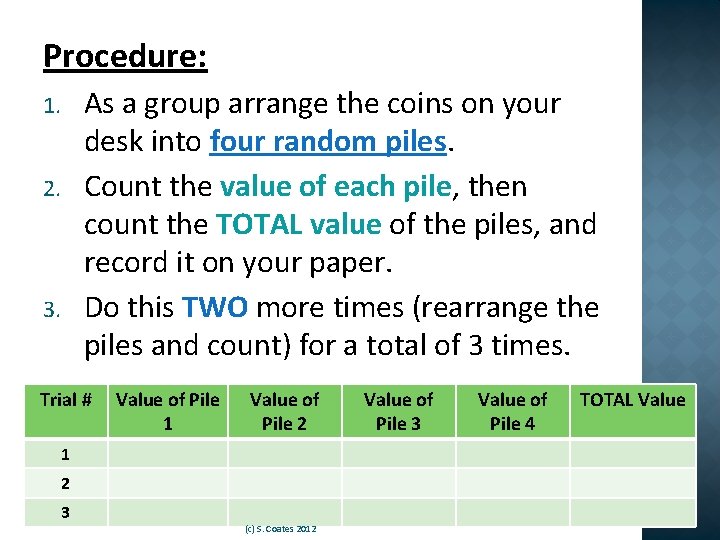
Procedure: As a group arrange the coins on your desk into four random piles. Count the value of each pile, then count the TOTAL value of the piles, and record it on your paper. Do this TWO more times (rearrange the piles and count) for a total of 3 times. 1. 2. 3. Trial # Value of Pile 1 Value of Pile 2 1 2 3 (c) S. Coates 2012 Value of Pile 3 Value of Pile 4 TOTAL Value

UNDERSTANDING CHEMICAL REACTIONS �Analysis: Did rearranging the coins change any individual coin? 2. Did rearranging the coins change the TOTAL value of the coins? 3. If you think of the coins as representing different types of atoms, what does this activity suggest about chemical reactions? 1. (c) S. Coates 2012

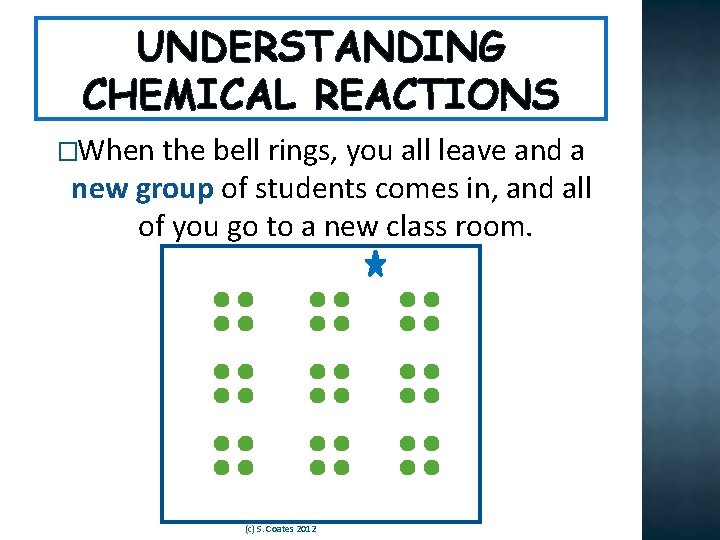
UNDERSTANDING CHEMICAL REACTIONS �Think about our class. We have a group of students and a teacher. (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS �When the bell rings, you all leave and a new group of students comes in, and all of you go to a new class room. (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS � The number of students and teachers in the school has not changed. But their arrangement is different and the new groups interact differently. (c) S. Coates 2012

WRITING CHEMICAL REACTIONS �We use symbols when writing chemical reactions. �Using these symbols makes it easier and faster to read and write reactions. �How else do you use symbols as a short-cut in daily life? (c) S. Coates 2012

WHAT DO THESE SYMBOLS MEAN? (c) S. Coates 2012

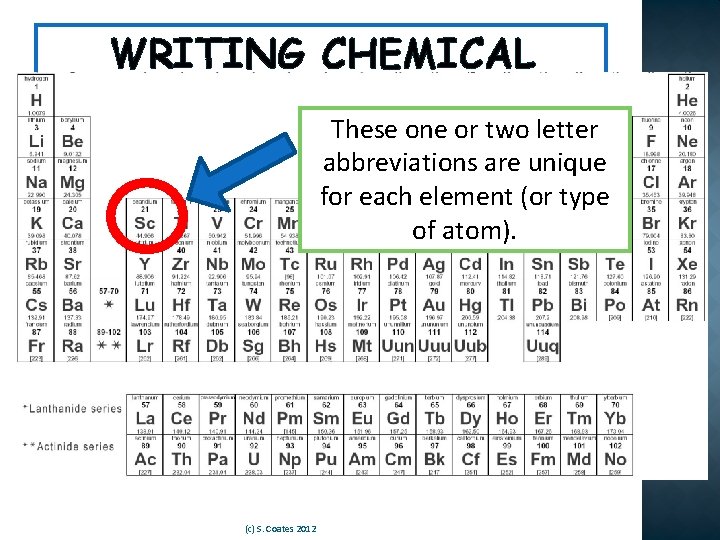
WRITING CHEMICAL REACTIONS These one or two letter abbreviations are unique �A chemical equation is a shorter, for each element (or type easier way to show chemical reactions, of atom). using symbols instead of words. �These symbols come from the Periodic Table of the Elements. (c) S. Coates 2012

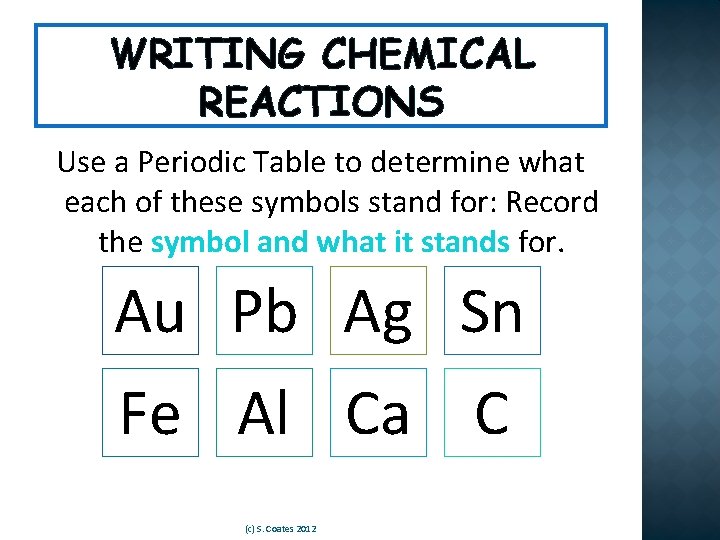
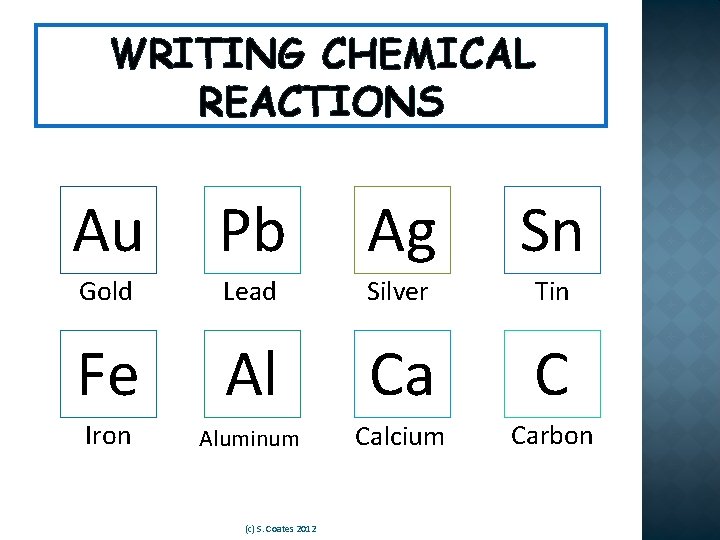
WRITING CHEMICAL REACTIONS Use a Periodic Table to determine what each of these symbols stand for: Record the symbol and what it stands for. Au Pb Ag Sn Fe Al Ca C (c) S. Coates 2012

WRITING CHEMICAL REACTIONS Au Pb Ag Sn Gold Lead Silver Tin Fe Al Ca C Iron Aluminum Calcium Carbon (c) S. Coates 2012

WRITING CHEMICAL REACTIONS �You have already seen many chemical formulas… H 2 O Hydrogen �What does the “O” stand for? Oxygen �What does the little “ 2” stand for? There are TWO hydrogen atoms �What does the “H” stand for? (c) S. Coates 2012

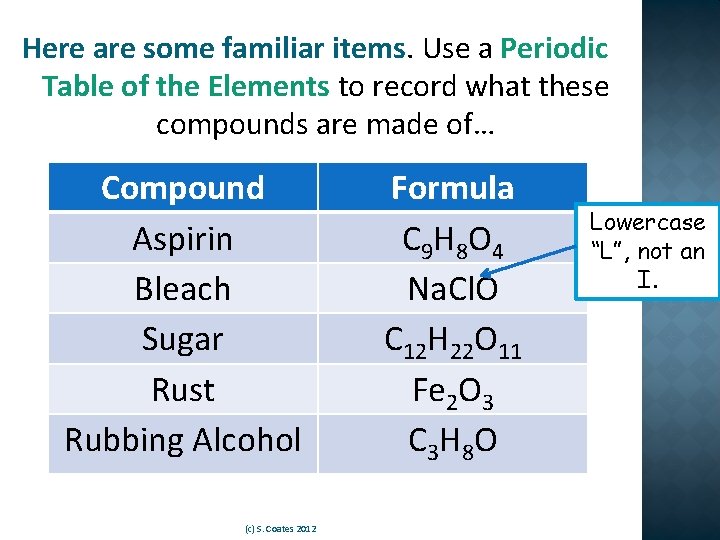
Here are some familiar items. Use a Periodic Table of the Elements to record what these compounds are made of… Compound Aspirin Bleach Sugar Rust Rubbing Alcohol (c) S. Coates 2012 Formula C 9 H 8 O 4 Na. Cl. O C 12 H 22 O 11 Fe 2 O 3 C 3 H 8 O Lowercase “L”, not an I.

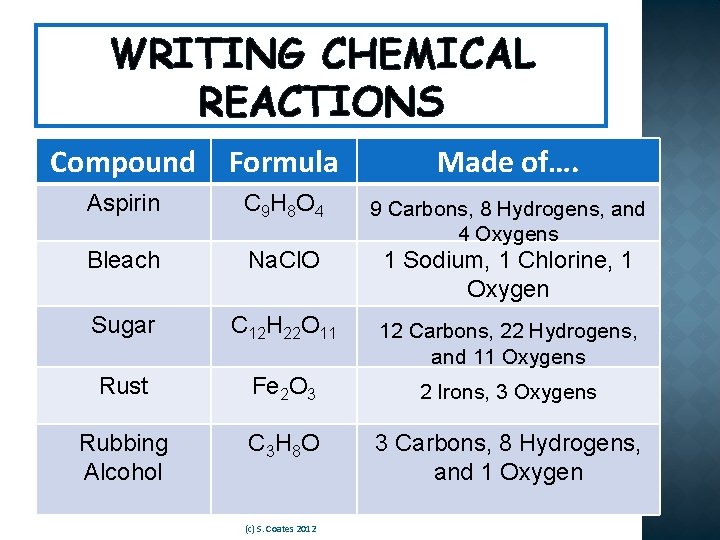
WRITING CHEMICAL REACTIONS Compound Formula Made of…. Aspirin C 9 H 8 O 4 9 Carbons, 8 Hydrogens, and 4 Oxygens Bleach Na. Cl. O 1 Sodium, 1 Chlorine, 1 Oxygen Sugar C 12 H 22 O 11 12 Carbons, 22 Hydrogens, and 11 Oxygens Rust Fe 2 O 3 2 Irons, 3 Oxygens Rubbing Alcohol C 3 H 8 O 3 Carbons, 8 Hydrogens, and 1 Oxygen (c) S. Coates 2012

WRITING CHEMICAL REACTIONS �Structure of a chemical equation: Every chemical equation is written THE SAME WAY… Reactant + Reactant Product + Product Sometimes there are more than 2 reactants. Sometimes there is only 1 reactant. Sometimes there are more than 2 products. Sometimes there is only 1 product. (c) S. Coates 2012

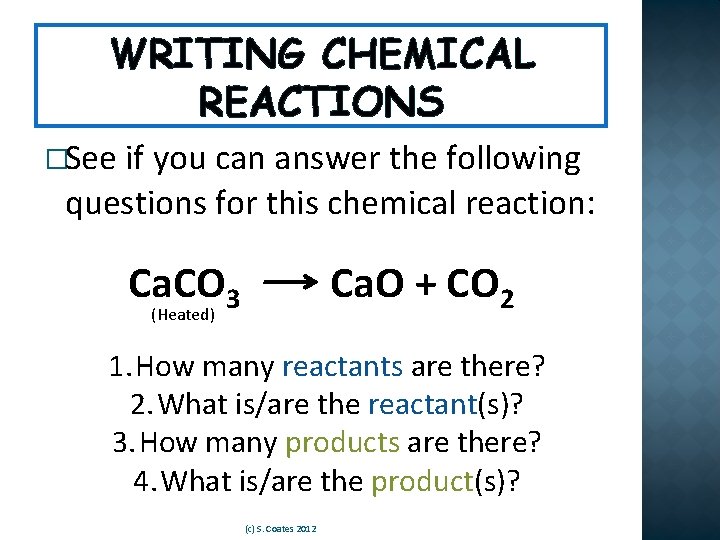
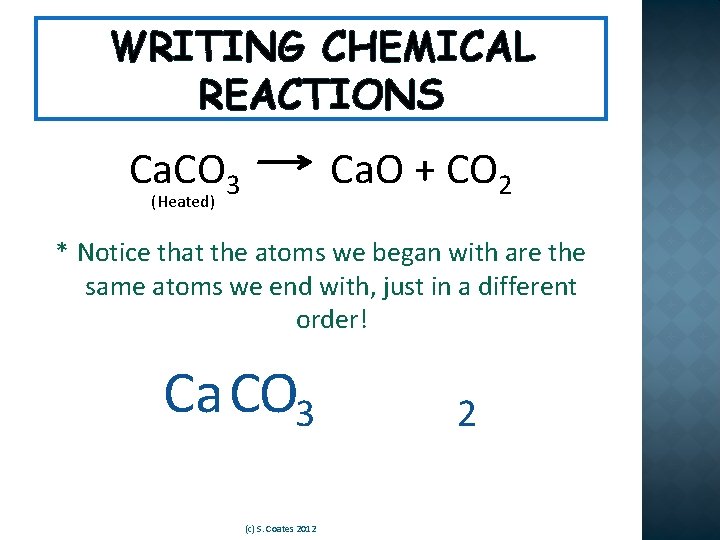
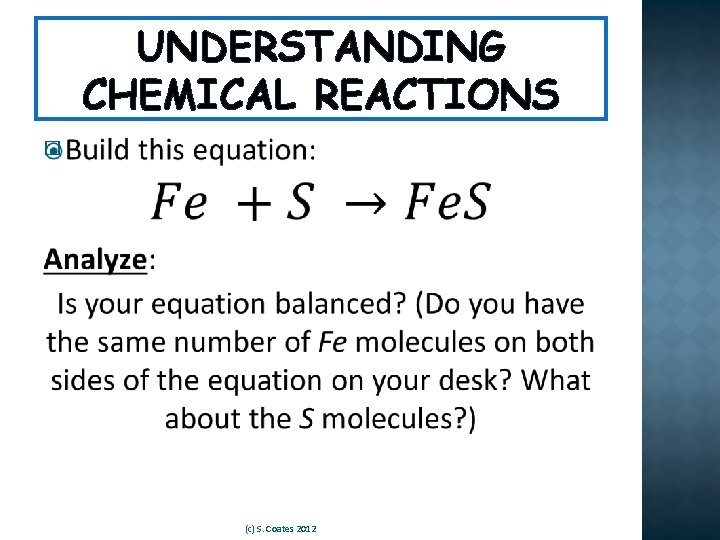
WRITING CHEMICAL REACTIONS �See if you can answer the following questions for this chemical reaction: Ca. CO 3 Ca. O + CO 2 (Heated) 1. How many reactants are there? 2. What is/are the reactant(s)? 3. How many products are there? 4. What is/are the product(s)? (c) S. Coates 2012

WRITING CHEMICAL REACTIONS Ca. CO 3 Ca. O + CO 2 (Heated) * Notice that the atoms we began with are the same atoms we end with, just in a different order! Ca CO 3 (c) S. Coates 2012 2

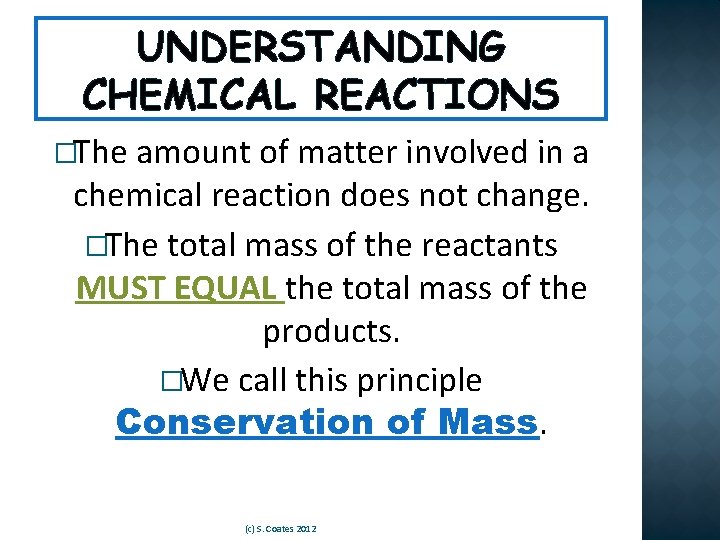
UNDERSTANDING CHEMICAL REACTIONS �The amount of matter involved in a chemical reaction does not change. �The total mass of the reactants MUST EQUAL the total mass of the products. �We call this principle Conservation of Mass. (c) S. Coates 2012

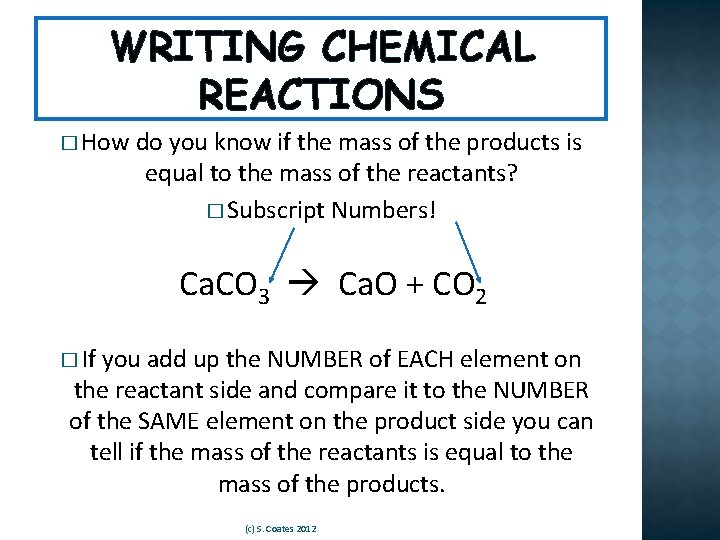
WRITING CHEMICAL REACTIONS � How do you know if the mass of the products is equal to the mass of the reactants? � Subscript Numbers! Ca. CO 3 Ca. O + CO 2 � If you add up the NUMBER of EACH element on the reactant side and compare it to the NUMBER of the SAME element on the product side you can tell if the mass of the reactants is equal to the mass of the products. (c) S. Coates 2012

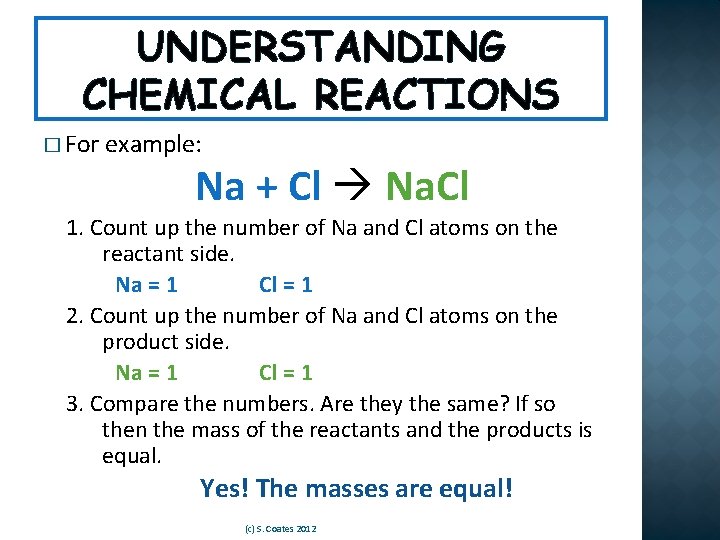
UNDERSTANDING CHEMICAL REACTIONS � For example: Na + Cl Na. Cl 1. Count up the number of Na and Cl atoms on the reactant side. Na = 1 Cl = 1 2. Count up the number of Na and Cl atoms on the product side. Na = 1 Cl = 1 3. Compare the numbers. Are they the same? If so then the mass of the reactants and the products is equal. Yes! The masses are equal! (c) S. Coates 2012

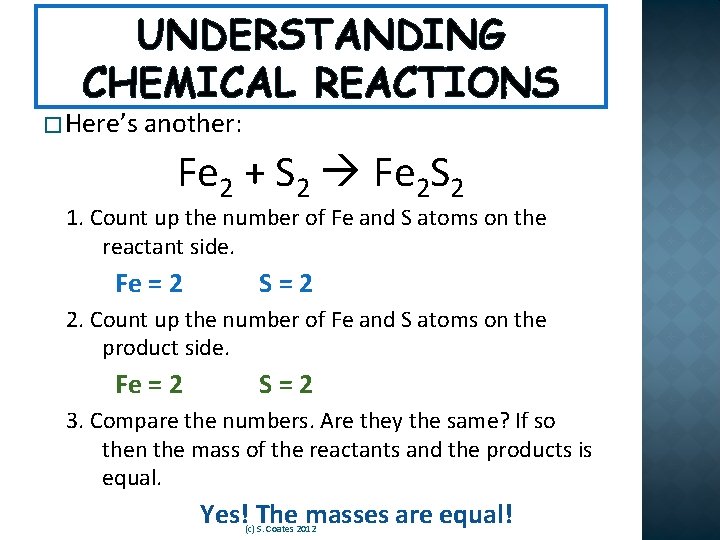
UNDERSTANDING CHEMICAL REACTIONS � Here’s another: Fe 2 + S 2 Fe 2 S 2 1. Count up the number of Fe and S atoms on the reactant side. Fe = 2 S=2 2. Count up the number of Fe and S atoms on the product side. Fe = 2 S=2 3. Compare the numbers. Are they the same? If so then the mass of the reactants and the products is equal. Yes! The masses are equal! (c) S. Coates 2012

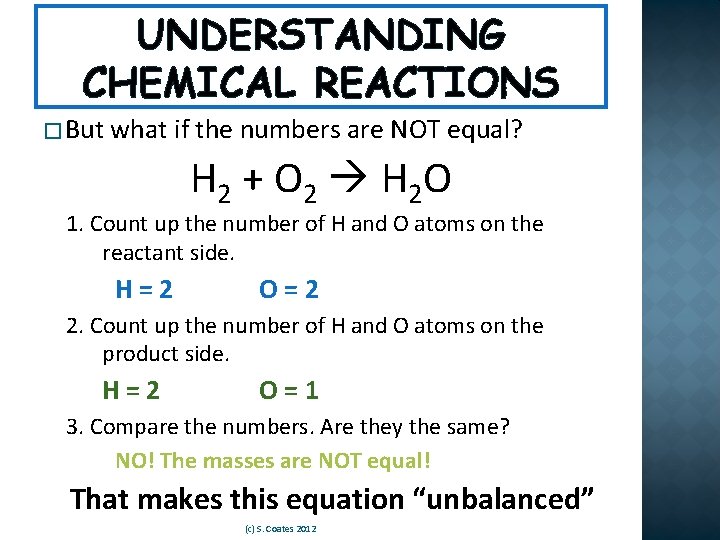
UNDERSTANDING CHEMICAL REACTIONS � But what if the numbers are NOT equal? H 2 + O 2 H 2 O 1. Count up the number of H and O atoms on the reactant side. H=2 O=2 2. Count up the number of H and O atoms on the product side. H=2 O=1 3. Compare the numbers. Are they the same? NO! The masses are NOT equal! That makes this equation “unbalanced” (c) S. Coates 2012

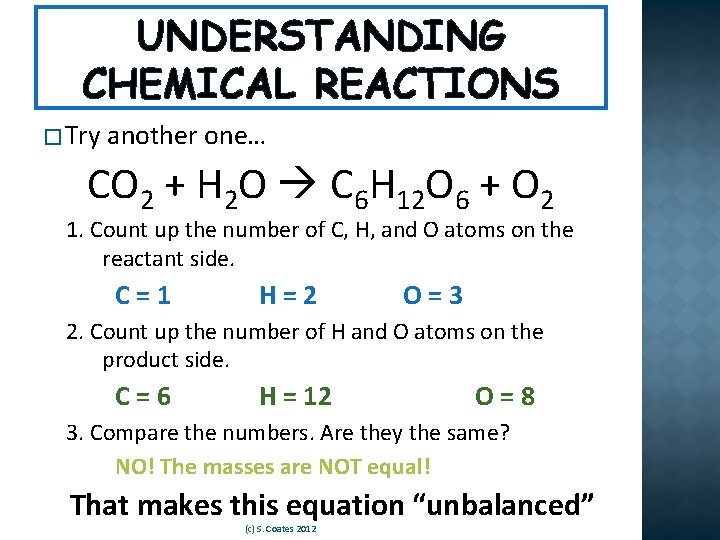
UNDERSTANDING CHEMICAL REACTIONS � Try another one… CO 2 + H 2 O C 6 H 12 O 6 + O 2 1. Count up the number of C, H, and O atoms on the reactant side. C=1 H=2 O=3 2. Count up the number of H and O atoms on the product side. C=6 H = 12 O=8 3. Compare the numbers. Are they the same? NO! The masses are NOT equal! That makes this equation “unbalanced” (c) S. Coates 2012

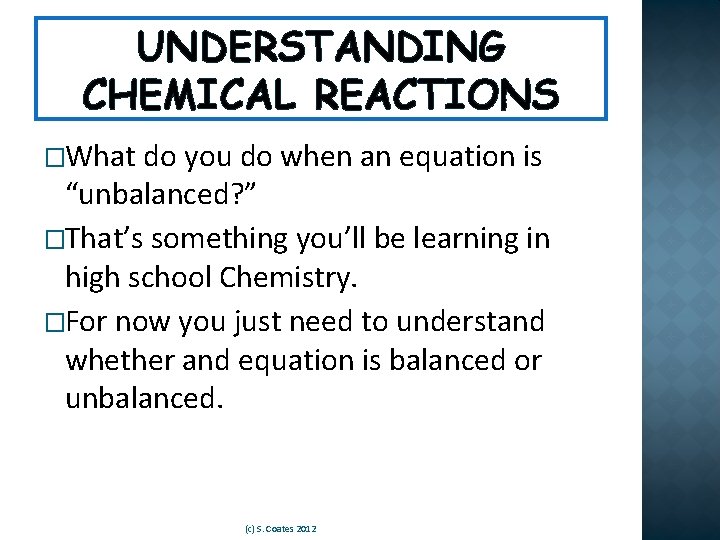
UNDERSTANDING CHEMICAL REACTIONS �What do you do when an equation is “unbalanced? ” �That’s something you’ll be learning in high school Chemistry. �For now you just need to understand whether and equation is balanced or unbalanced. (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS �Activity! “Is it Balanced? ” �Materials Needed: � 1 envelop of activity cards per group (c) S. Coates 2012

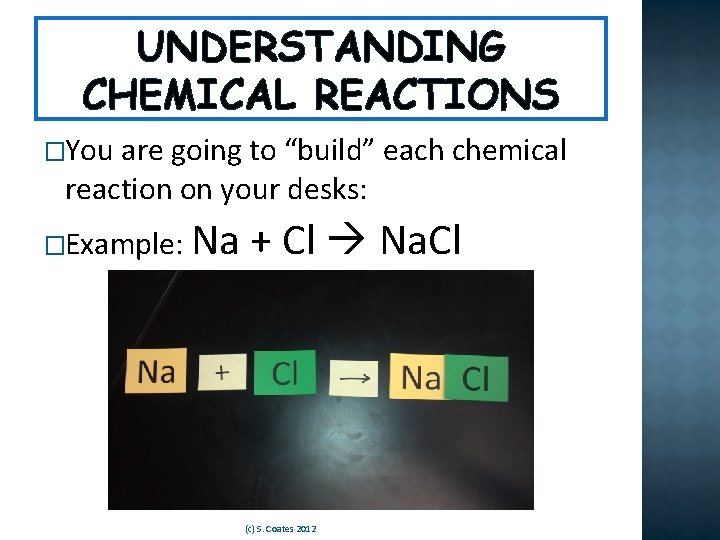
UNDERSTANDING CHEMICAL REACTIONS �You are going to “build” each chemical reaction on your desks: �Example: Na + Cl Na. Cl (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS � (c) S. Coates 2012

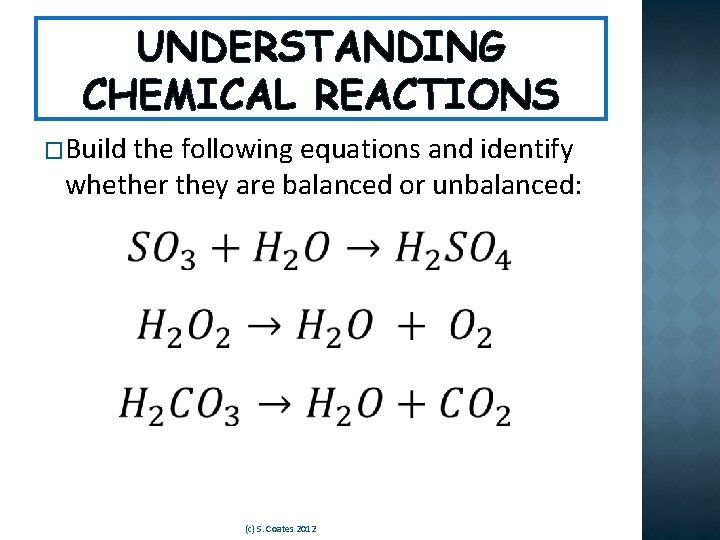
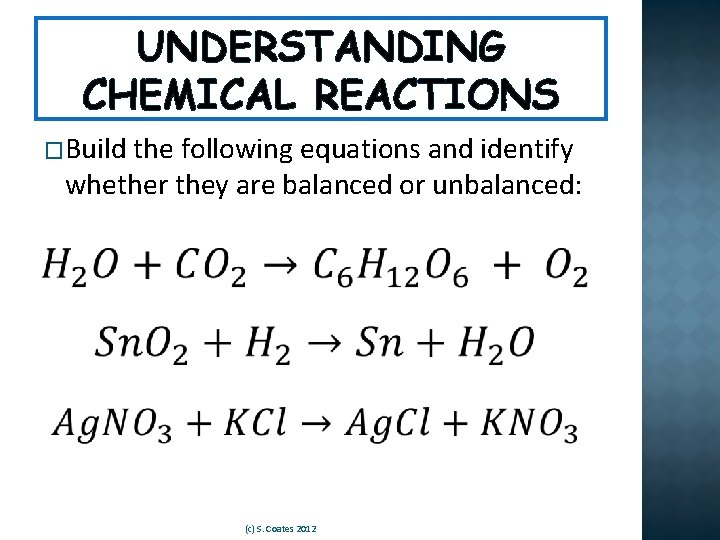
UNDERSTANDING CHEMICAL REACTIONS �Build the following equations and identify whether they are balanced or unbalanced: (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS �Now you can practice on your own! �Do the “Chemical Reactions” worksheet. �This will be due: _______ (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS �Build the following equations and identify whether they are balanced or unbalanced: (c) S. Coates 2012

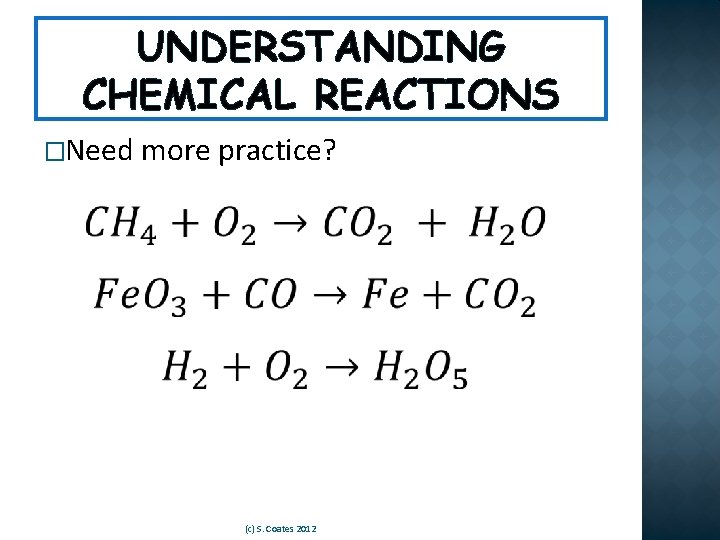
UNDERSTANDING CHEMICAL REACTIONS �Need more practice? (c) S. Coates 2012

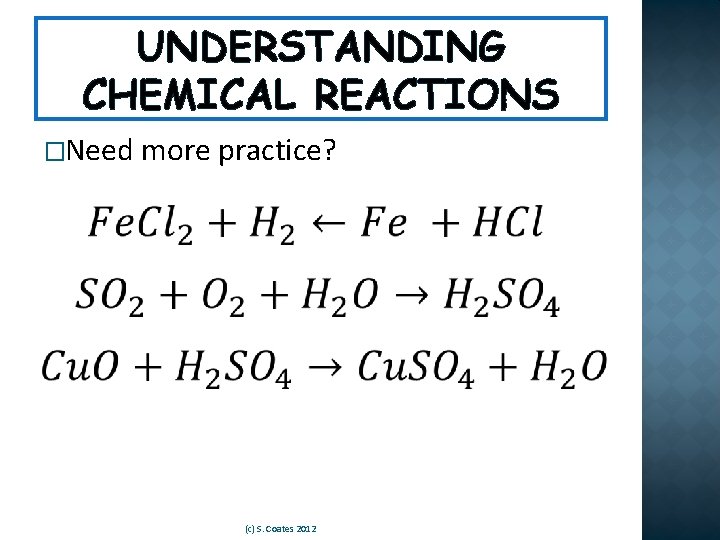
UNDERSTANDING CHEMICAL REACTIONS �Need more practice? (c) S. Coates 2012

UNDERSTANDING CHEMICAL REACTIONS �Let’s summarize: �Chemical reactions can cause changes in properties and changes in energy. �Chemical reactions occur when the bonds between atoms break, or when new bonds between atoms are formed. �We can write equations for chemical reactions by using symbols �Chemical reactions must follow the Law of Conservation of Mass. (c) S. Coates 2012