C 1 Atomic Structure and the Periodic Table
C 1: Atomic Structure and the Periodic Table Key Concepts

Definitions: Element: a pure substance in which all of the atoms are the same. Compound: two or more elements chemically bonded. Mixture: two or more elements or compounds mixed together but not chemically combined. Molecule: two or more atoms covalently bonded together to form The smallest unit of an element or compound.
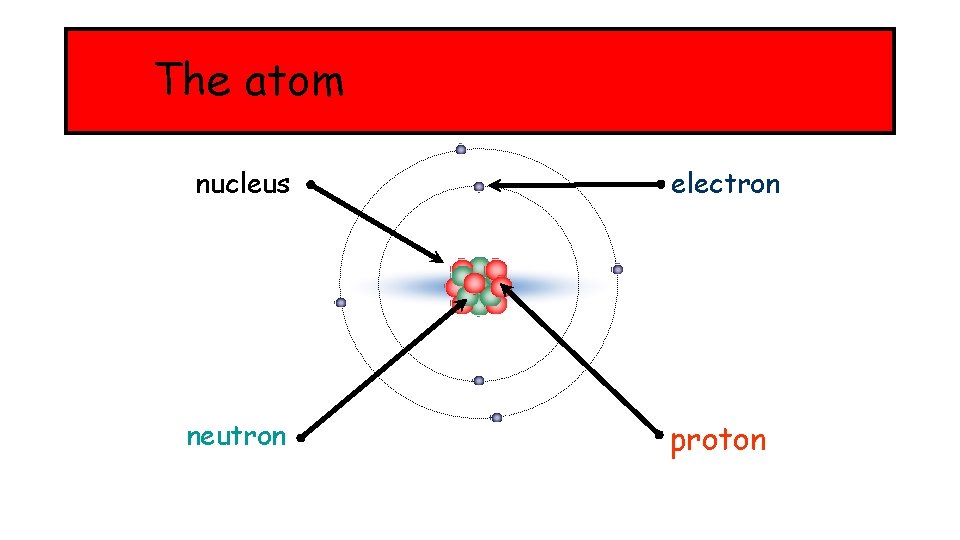
The atom nucleus electron neutron proton

Mass and electrical charge There are two properties of protons, neutrons and electrons that are especially important: l mass l electrical charge. Particle Mass Charge proton 1 +1 neutron 1 0 electron almost 0 -1 The atoms of an element contain equal numbers of protons and electrons and so have no overall charge.

How are electrons arranged? The arrangement of electrons in these shells is often called the electron configuration. 1 st shell 2 nd shell 1 st shell holds a maximum of 2 electrons. 2 nd shell holds a maximum of 8 electrons. 3 rd shell holds a maximum of 8 electrons.
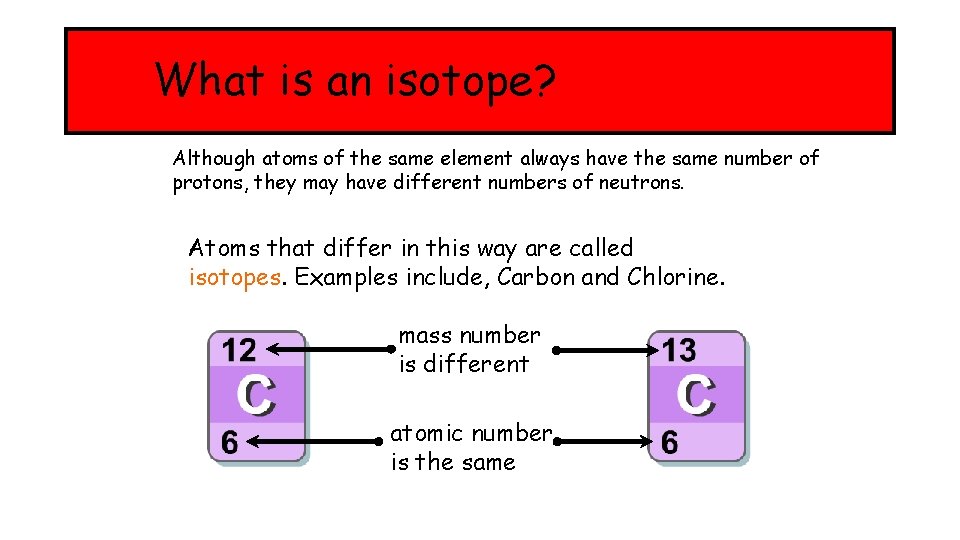
What is an isotope? Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that differ in this way are called isotopes. Examples include, Carbon and Chlorine. mass number is different atomic number is the same

Trends in Chemical Reactivity Reactions all involve the loss of the outermost electron which changes the metal atom into a metal 1+ ion. Losing this electron seems to get easier as we go down the group. Li Na K Rb Cs Reactivity Increases Reactivity increases down the group.
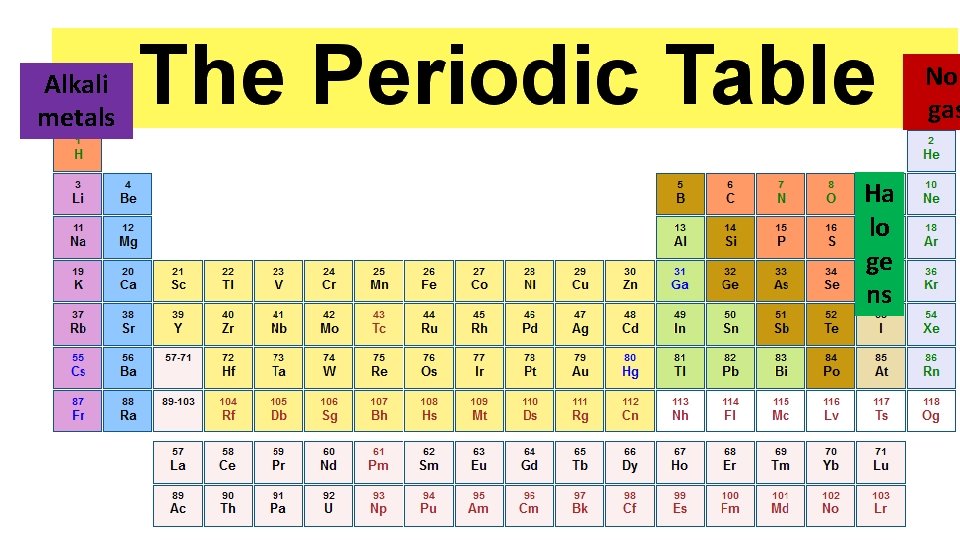
Nob gas Alkali metals Ha lo ge ns
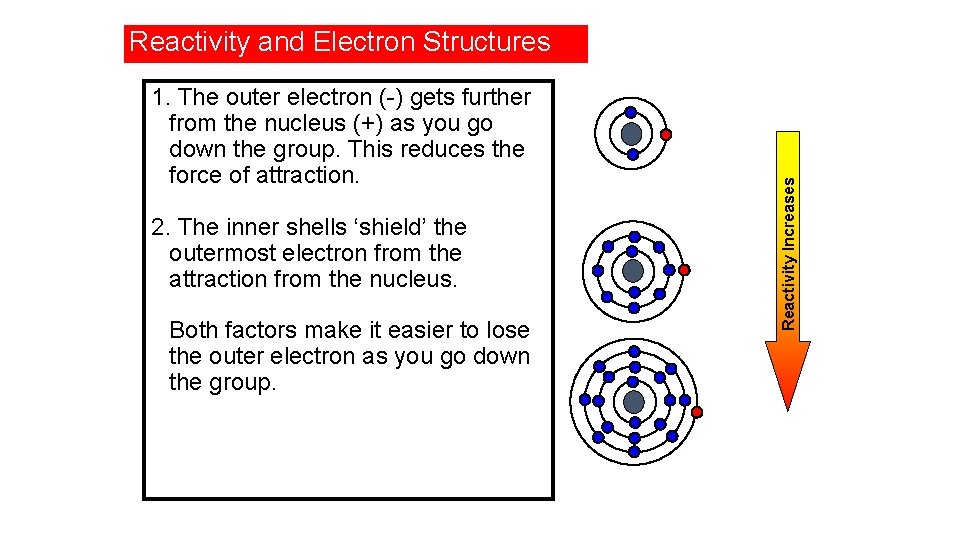
1. The outer electron (-) gets further from the nucleus (+) as you go down the group. This reduces the force of attraction. 2. The inner shells ‘shield’ the outermost electron from the attraction from the nucleus. Both factors make it easier to lose the outer electron as you go down the group. Reactivity Increases Reactivity and Electron Structures
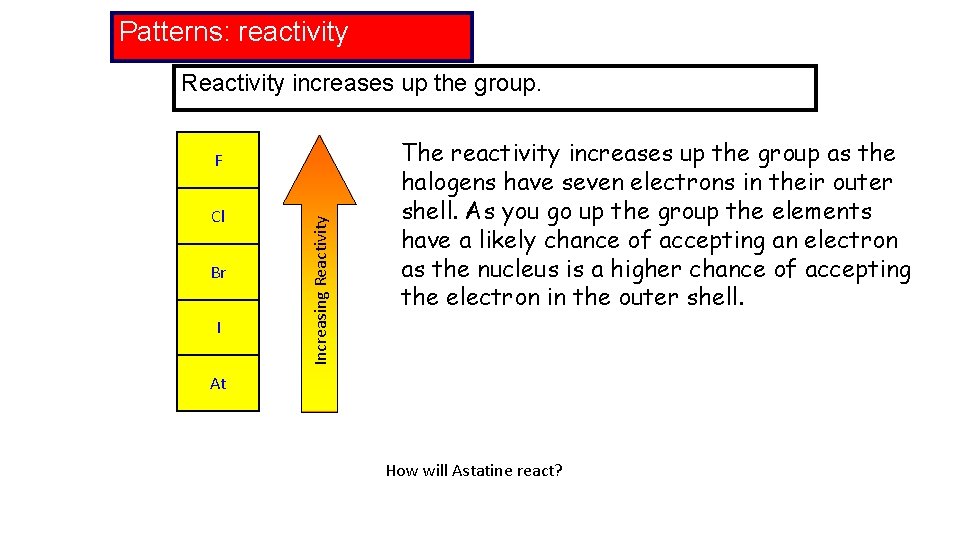
Patterns: reactivity Reactivity increases up the group. Cl Br I Increasing Reactivity F The reactivity increases up the group as the halogens have seven electrons in their outer shell. As you go up the group the elements have a likely chance of accepting an electron as the nucleus is a higher chance of accepting the electron in the outer shell. At How will Astatine react?
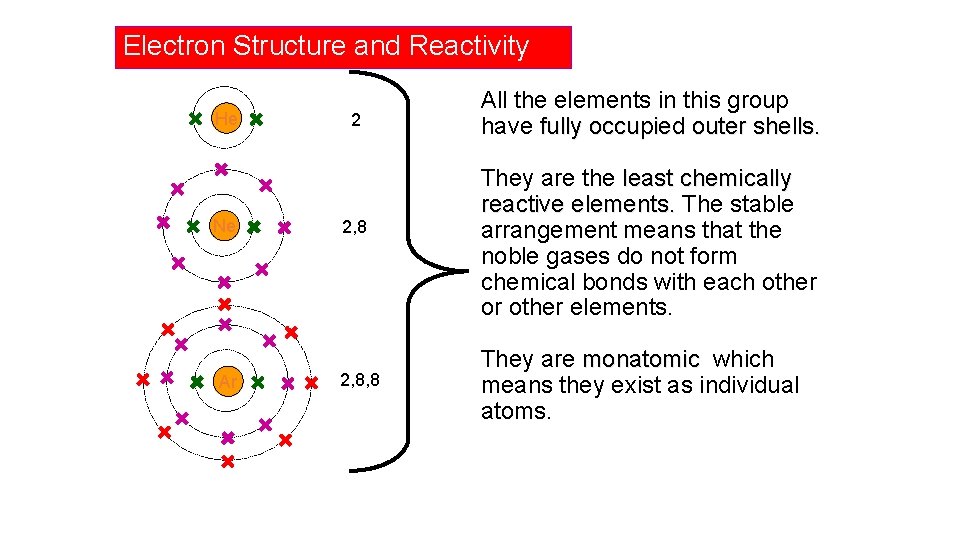
Electron Structure and Reactivity He Ne Ar 2 2, 8, 8 All the elements in this group have fully occupied outer shells. They are the least chemically reactive elements. The stable arrangement means that the noble gases do not form chemical bonds with each other or other elements. They are monatomic which means they exist as individual atoms.
- Slides: 11