Building the inHouse Review Competence of GRP via

Building the “in-House Review” Competence of GRP via an Innovative Review Model in Chinese Taipei Meir-Chyun Tzou, Ph. D. Director, Division of Drugs & New Biotechnology Products, TFDA

Outline Organization of TFDA v Reform of Taiwan Drug Review System v Implementation of Good Review Practice ØKey elements of GRP v Ø Template and tools Ø Reviewer training Ø Qualification of reviewer v Case 2 study

Establishment of Taiwan FDA v Taiwan FDA (TFDA) was inaugurated on Jan. 1, 2010 v TFDA supersedes the following 4 bureaus of Department of Health Ø Bureau of Food Safety Ø Bureau of Pharmaceutical Affairs Ø Bureau of Food and Drug Analysis Ø Bureau of Controlled Drugs 3 3
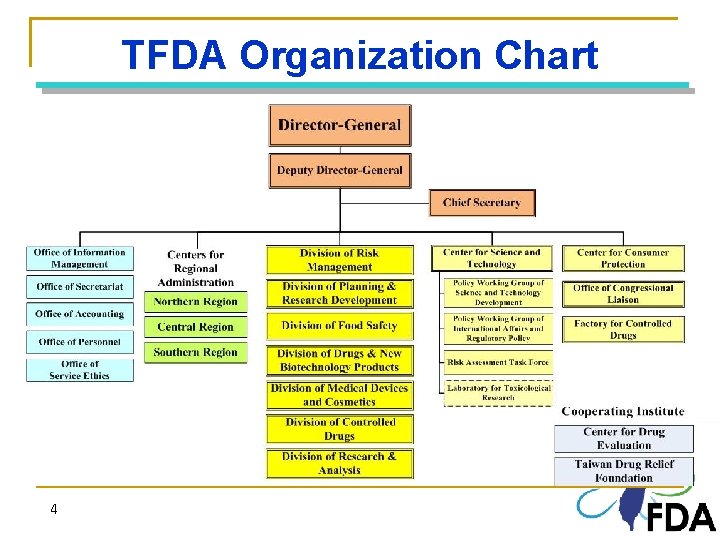
TFDA Organization Chart 4 4
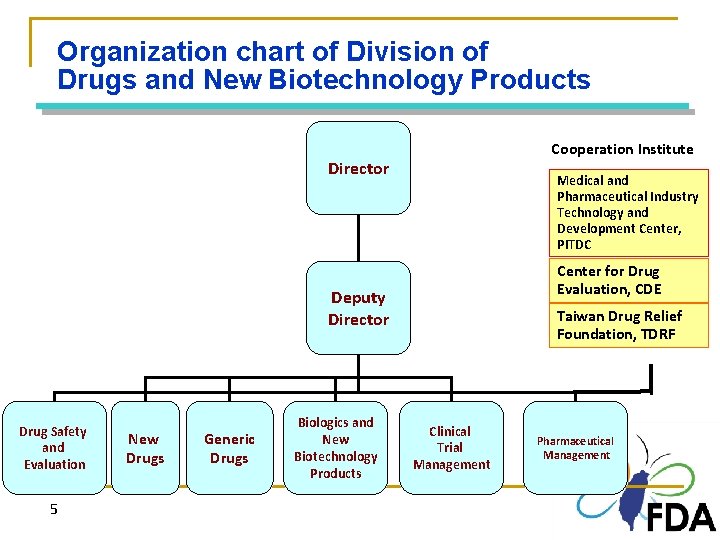
Organization chart of Division of Drugs and New Biotechnology Products Cooperation Institute Director Medical and Pharmaceutical Industry Technology and Development Center, PITDC Center for Drug Evaluation, CDE Deputy Director Drug Safety and Evaluation 5 New Drugs Generic Drugs Biologics and New Biotechnology Products 5 Taiwan Drug Relief Foundation, TDRF Clinical Trial Management Pharmaceutical Management
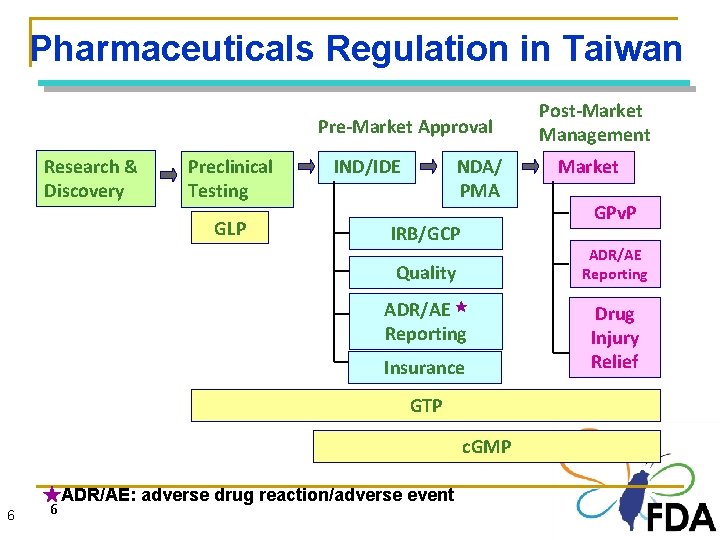
Pharmaceuticals Regulation in Taiwan Pre-Market Approval Research & Discovery Preclinical Testing IND/IDE GLP NDA/ PMA IRB/GCP ADR/AE ★ Reporting Insurance GTP c. GMP 6 6 6 Market GPv. P ADR/AE Reporting Quality ★ADR/AE: adverse drug reaction/adverse event Post-Market Management Drug Injury Relief

Reform of Taiwan Drug Review System v Rationalization of the Review System Transparent , Unified, Fast 7 v Regulation Strategies v post-marketing surveillance
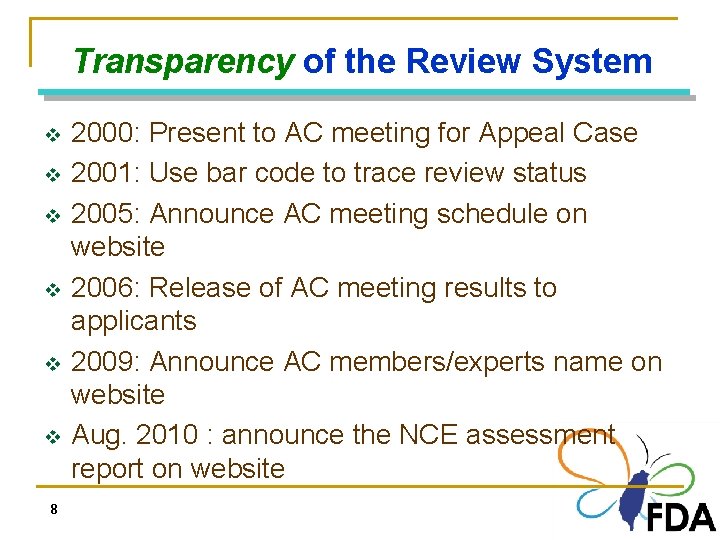
Transparency of the Review System v v v 8 2000: Present to AC meeting for Appeal Case 2001: Use bar code to trace review status 2005: Announce AC meeting schedule on website 2006: Release of AC meeting results to applicants 2009: Announce AC members/experts name on website Aug. 2010 : announce the NCE assessment report on website 8

Transparency and Quality Assurance Implementation of Good Review Practice (GRP) v Review quality assurance: QA/QC task force v On-line Roadmap : for tracking review progress, starting from May 2010 v 9

Unified Drug Review System v TFDA Review Team Ø TFDA staff + CDE ★ reviewers Ø Responsible for drugs and medical devices review Ø General cases & fast-track review process v Advisory Committee (AC) Ø Committee members from academics, research organizations and health institutes Ø Provides TFDA related consultation and advices Ø Special cases review ★ 10 Center of Drug Evaluation (CDE) was Established by the DOH (Department of Health) in 1998 as a NGO, NPO. Its mission is to assist DOH to evaluate new drugs and new medical devices for regulatory requirements and offer related consultation services. 10

Reform of the Advisory Committee Duties of the Advisory Committee v Special case review Ø Clinical Trial :First in human, Ethnic and Ethical Concern and etc. Ø NDA:Global New, NCE not approve by US FDA and EMA, Botanical product, Biosimilar and etc. v 11 Ethics, Public Health and Public awareness issues 11

Innovative Review Model of TFDA/CDE/AC v v 12 Create a functional “TFDA office for Evaluation” with part of CDE reviewers sit into the TFDA office building-”In House Review” and joint training program Flexibility of CDE: funding resource, salary, head count, science based technical support from regulatory consultation to review Authority of TFDA: legal, policy position Streamline the review process
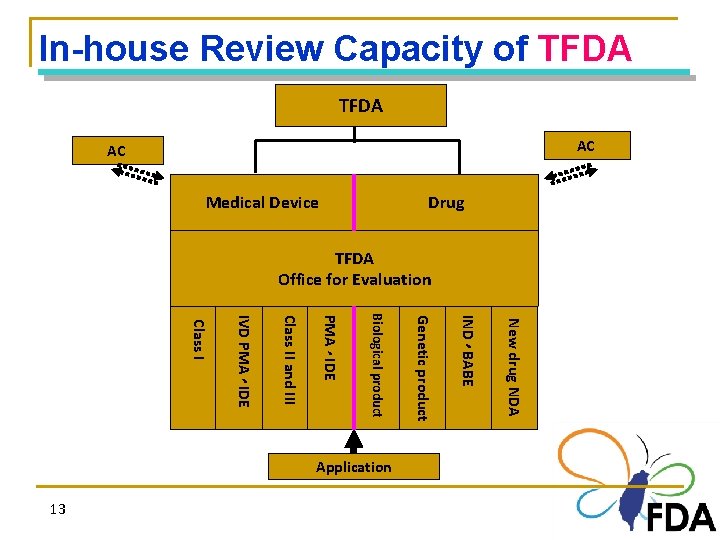
In-house Review Capacity of TFDA AC AC Medical Device Drug TFDA Office for Evaluation 、 New drug NDA 13 IND BABE Application Genetic product Biological product PMA IDE Class II and III IVD PMA IDE Class I 、 、
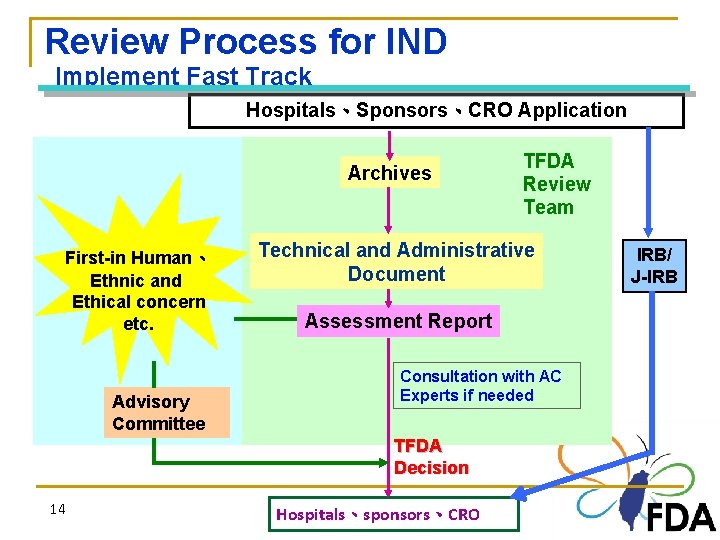
Review Process for IND Implement Fast Track Hospitals、Sponsors、CRO Application Archives First-in Human、 Ethnic and Ethical concern etc. Advisory Committee Technical and Administrative Document Assessment Report Consultation with AC Experts if needed TFDA Decision 14 TFDA Review Team Hospitals、sponsors、CRO 14 IRB/ J-IRB
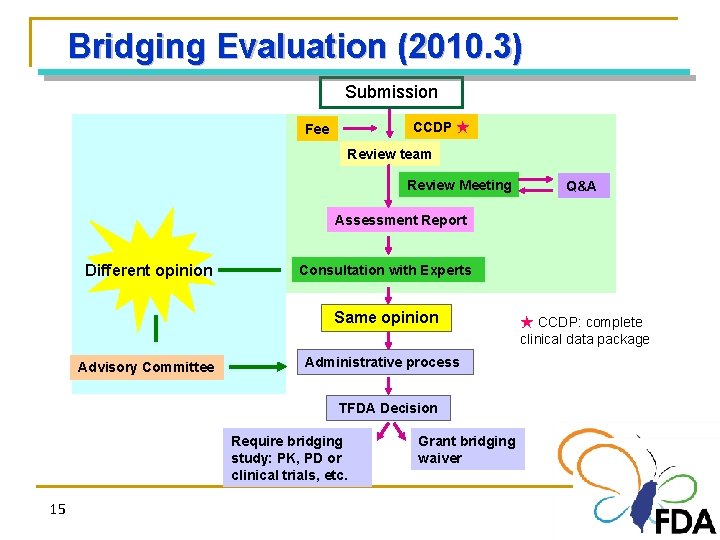
Bridging Evaluation (2010. 3) Submission CCDP ★ Fee Review team Review Meeting Q&A Assessment Report Different opinion Consultation with Experts Same opinion Advisory Committee Administrative process TFDA Decision Require bridging study: PK, PD or clinical trials, etc. 15 15 Grant bridging waiver ★ CCDP: complete clinical data package
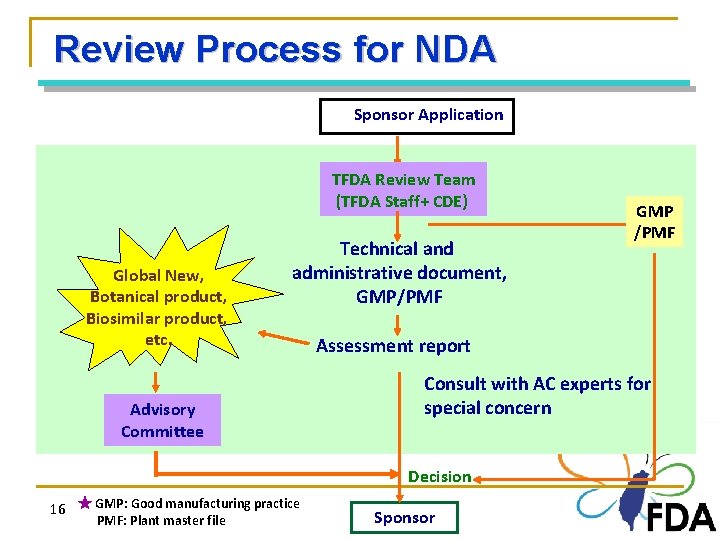
Review Process for NDA Sponsor Application TFDA Review Team (TFDA Staff+ CDE) Global New, Botanical product, Biosimilar product, etc. Technical and administrative document, GMP/PMF Assessment report Consult with AC experts for special concern Advisory Committee Decision 16 ★ GMP: Good manufacturing practice PMF: Plant master file GMP /PMF 16 Sponsor
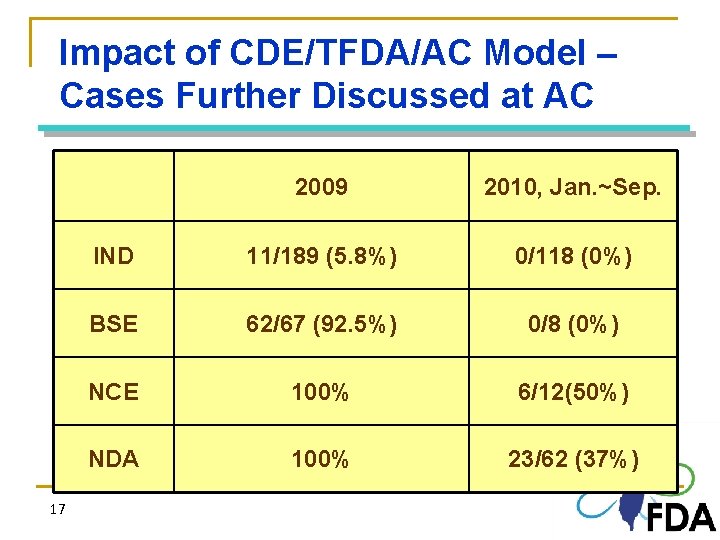
Impact of CDE/TFDA/AC Model – Cases Further Discussed at AC 17 2009 2010, Jan. ~Sep. IND 11/189 (5. 8%) 0/118 (0%) BSE 62/67 (92. 5%) 0/8 (0%) NCE 100% 6/12(50%) NDA 100% 23/62 (37%)

Implementation of Good Review Practice (GRP) 18

Key elements of GRP Template and tools v Reviewer training v Qualification of reviewer v 19
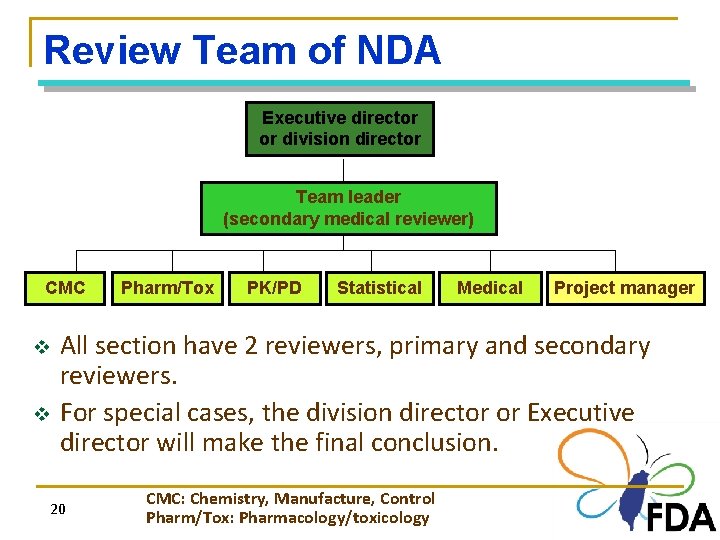
Review Team of NDA Executive director or division director Team leader (secondary medical reviewer) CMC v v Pharm/Tox PK/PD Statistical Medical Project manager All section have 2 reviewers, primary and secondary reviewers. For special cases, the division director or Executive director will make the final conclusion. 20 CMC: Chemistry, Manufacture, Control Pharm/Tox: Pharmacology/toxicology
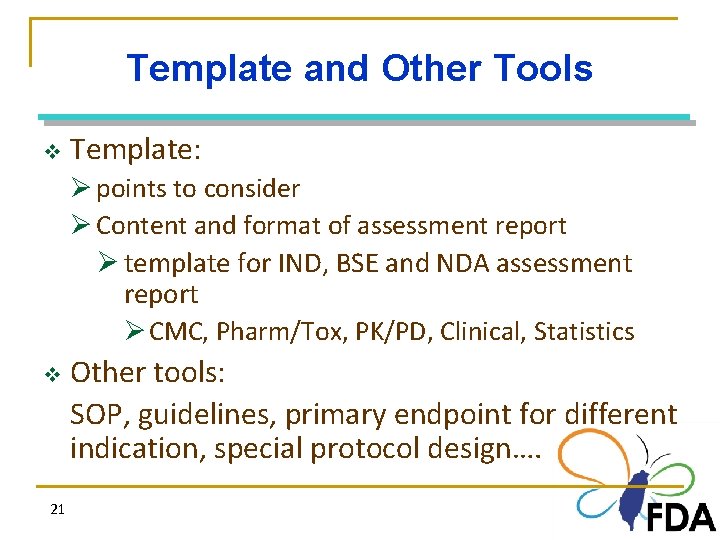
Template and Other Tools v Template: Ø points to consider Ø Content and format of assessment report Ø template for IND, BSE and NDA assessment report Ø CMC, Pharm/Tox, PK/PD, Clinical, Statistics v 21 Other tools: SOP, guidelines, primary endpoint for different indication, special protocol design….

Template examplekey elements in IND review-clinical section 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 22 Rationale and expected value of the study Protection of the welfare for all subjects Diagnosis of the subjects Inclusion and exclusion criteria Dose selection, administration route, and treatment duration Consideration and protection of the safety of subjects Limitations on concomitant medication usage Selection of therapeutic endpoints Sub-study Informed consent form
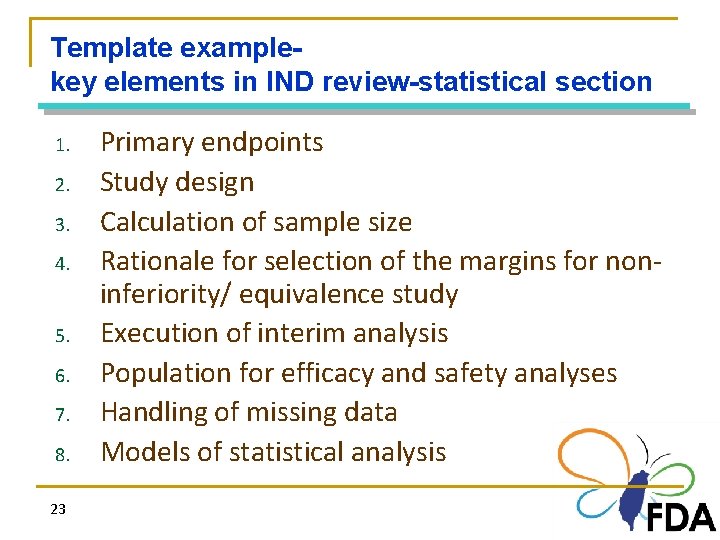
Template examplekey elements in IND review-statistical section 1. 2. 3. 4. 5. 6. 7. 8. 23 Primary endpoints Study design Calculation of sample size Rationale for selection of the margins for noninferiority/ equivalence study Execution of interim analysis Population for efficacy and safety analyses Handling of missing data Models of statistical analysis
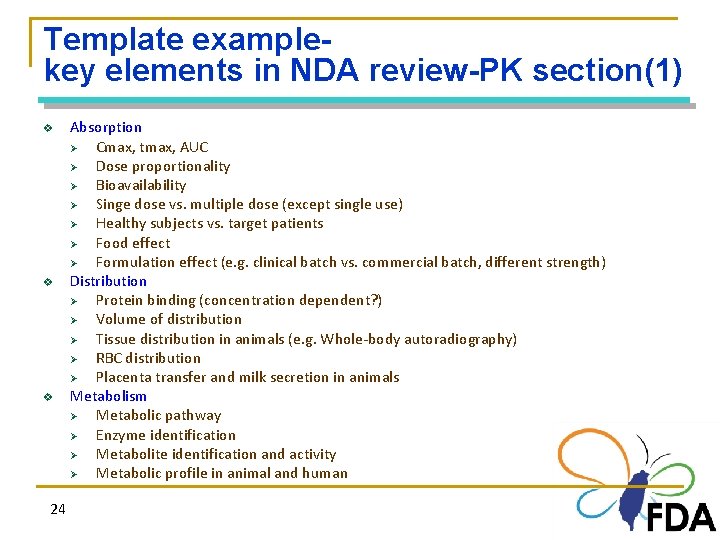
Template examplekey elements in NDA review-PK section(1) v v v 24 Absorption Ø Cmax, tmax, AUC Ø Dose proportionality Ø Bioavailability Ø Singe dose vs. multiple dose (except single use) Ø Healthy subjects vs. target patients Ø Food effect Ø Formulation effect (e. g. clinical batch vs. commercial batch, different strength) Distribution Ø Protein binding (concentration dependent? ) Ø Volume of distribution Ø Tissue distribution in animals (e. g. Whole-body autoradiography) Ø RBC distribution Ø Placenta transfer and milk secretion in animals Metabolism Ø Metabolic pathway Ø Enzyme identification Ø Metabolite identification and activity Ø Metabolic profile in animal and human
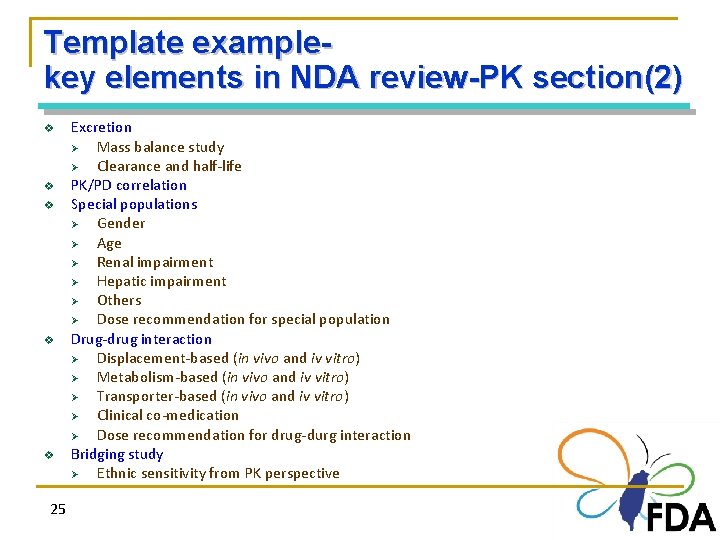
Template examplekey elements in NDA review-PK section(2) v v v 25 Excretion Ø Mass balance study Ø Clearance and half-life PK/PD correlation Special populations Ø Gender Ø Age Ø Renal impairment Ø Hepatic impairment Ø Others Ø Dose recommendation for special population Drug-drug interaction Ø Displacement-based (in vivo and iv vitro) Ø Metabolism-based (in vivo and iv vitro) Ø Transporter-based (in vivo and iv vitro) Ø Clinical co-medication Ø Dose recommendation for drug-durg interaction Bridging study Ø Ethnic sensitivity from PK perspective

Reviewer’s Training and Quality Control v Review team : Primary reviewer v v 26 Secondary reviewer supervisor Consultation with a group of 100 domestic experts and 5 oversea contracted consultants with FDA experience Regular case discussion, review guidance discussion and drafting Structured training and evaluation program for primary and secondary reviewers Internal/external QA/QC task force

Orientation course for new reviewer v v Basic course Advanced course Ø Ø Basic introduction for new drug development, CMC, pharmacology/toxicology, PK/PD, Statistics, Biologics, key element for IND review Computer skill, soft skill for consultation and communication Consultation process, IND process, NDA process, BSE process Review SOP Each new reviewer must complete the orientation course within 3 months 27

On- Job training v v v v 28 Real case training- supervised mainly under secondary reviewer Ø Learn how to set the approval criteria Case discussion with senior Advisory committee member once every week since 2005 Lectures from experts CDER-101 training course CBER-101 training course DIA meeting in USA, Japan, and Europe Optional training course Ø Presentation skill in English Ø Communication skill

Qualification of secondary reviewer At least 2 years’ experience as reviewer v Logic thinking, decision making, quality of assessment report, communication skill, leadership in team work are key elements to evaluate the qualification of secondary reviewer v Division Director and Executive Director are responsible to evaluate the secondary reviewer v 29

External QA/QC for GRP of TFDA/CDE’s Assessment Reports v v v 30 Annual satisfactory rate survey for stakeholders in soft skill and professional performance via independent CRO Stakeholders meeting with representatives from industry association. Give meeting minutes and follow up action report Consultation/IND/NDA assessment reports of all disciplines rountinely scrutinized in written by contracted external consultants with FDA experience in clinical, statistics, PK, Pharm/Tox. , CMC sections followed by face to face group discussion sessions
Pivotal Trial Study Design and Primary Endpoints Selection for Different Cancer Indication – An Internal Review for NDA 2004 -2008 For consistence in regulatory requirement v Review by cancer type, stage, with control group or not, effect size, endpoint selection like overall survival, progression free survival, disease free survival, time to progression, objective tumor response rate, surrogate endpoints, new indication, new formulation and approval or not v 31

Case study 32
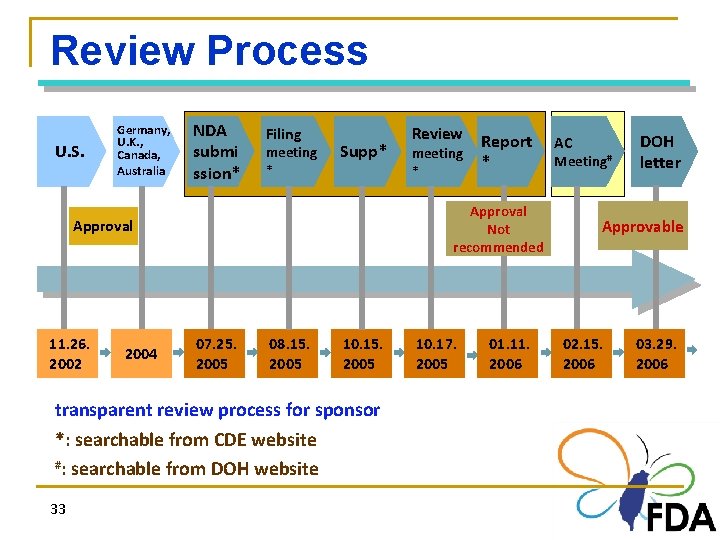
Review Process U. S. Germany, U. K. , Canada, Australia NDA submi ssion* Filing meeting * Supp* 2004 07. 25. 2005 08. 15. 2005 10. 15. 2005 transparent review process for sponsor *: searchable from CDE website #: searchable from DOH website 33 meeting * Report * Approval Not recommended Approval 11. 26. 2002 Review 10. 17. 2005 01. 11. 2006 AC Meeting# DOH letter Approvable 02. 15. 2006 03. 29. 2006
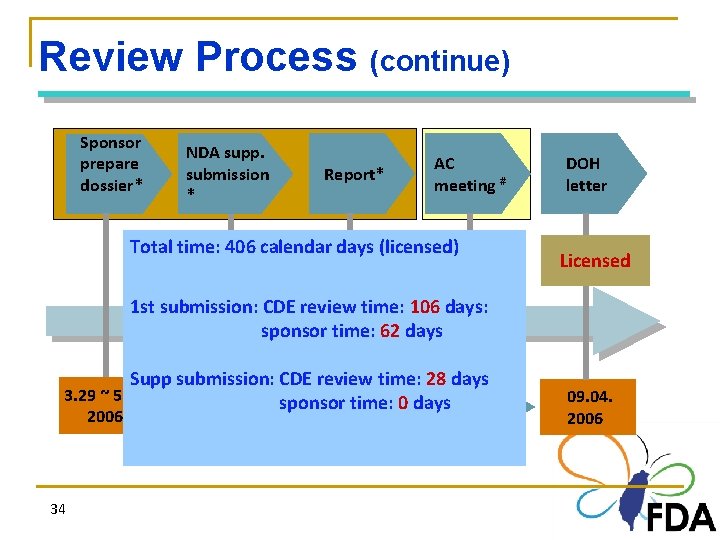
Review Process (continue) Sponsor prepare dossier* NDA supp. submission * Report* AC meeting # Total time: 406 calendar days (licensed) DOH letter Licensed 1 st submission: CDE review time: 106 days: sponsor time: 62 days Supp submission: CDE review time: 28 days 3. 29 ~ 5. 15 05. 15. sponsor 06. 12. time: 0 days 07. 12. 2006 34 2006 09. 04. 2006

IF NDA Atomoxetine Submitted in 2010 in Stead of 2005…. v v 35 Early submission with 1 CPP after FDA approval 2 years Shorter CDE/TFDA review time, administrative time: 30% Better interactions with CDE/TFDA in scientific advice, supplement information, labeling discussion, REMS Possibility of better drug price for clinical trial conduct in Chinese Taipei

Thank you for your attention 36
- Slides: 36