Building Blocks of Life An Introduction Carbon is
Building Blocks of Life An Introduction

Carbon is unparalleled in its ability to form large, complex, and diverse molecules • Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds • Carbon—The Backbone of Biological Molecules
�Electron configuration determines the kinds and number of bonds an atom will form with other atoms �With four valence electrons, carbon can form four covalent bonds with a variety of atoms ◦ makes large, complex molecules possible Carbon atoms can form diverse molecules by bonding to four other atoms
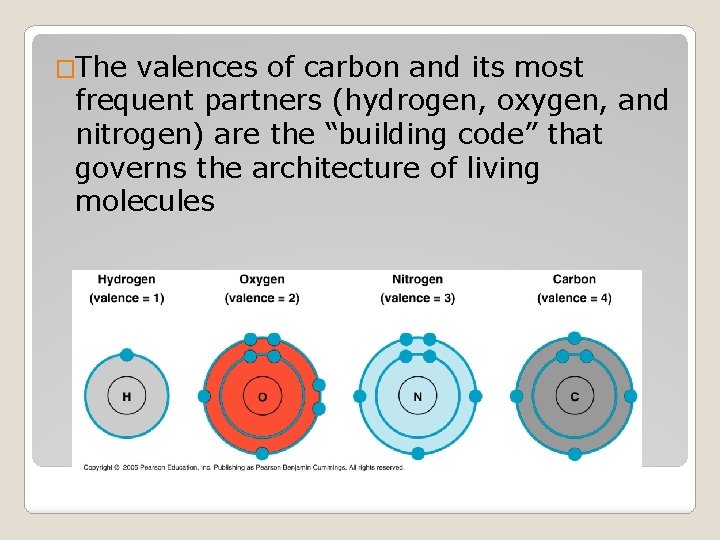
�The valences of carbon and its most frequent partners (hydrogen, oxygen, and nitrogen) are the “building code” that governs the architecture of living molecules
�Within cells, small organic molecules are joined together to form larger molecules �Macromolecules are large molecules composed of thousands of covalently connected atoms Macromolecules

Monomers build polymers linked together by covalent bonds • Three of the four classes of life’s organic molecules are polymers: • ◦ Carbohydrates ◦ Proteins ◦ Nucleic acids ◦ Lipids polymers built from monomers
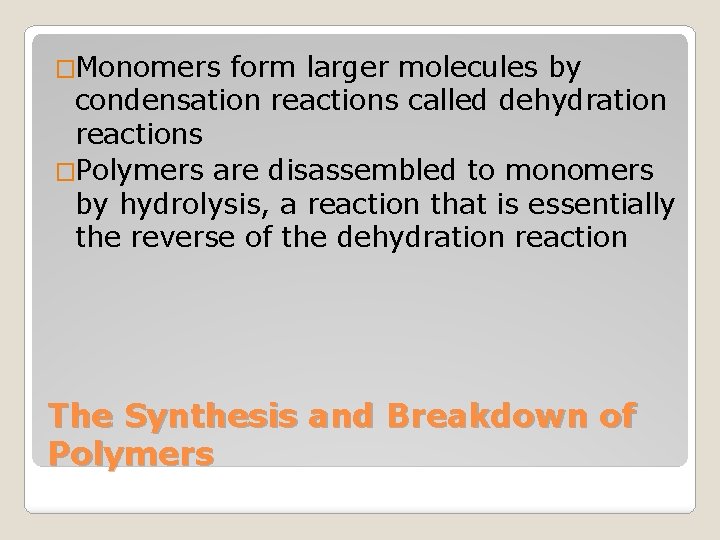
�Monomers form larger molecules by condensation reactions called dehydration reactions �Polymers are disassembled to monomers by hydrolysis, a reaction that is essentially the reverse of the dehydration reaction The Synthesis and Breakdown of Polymers
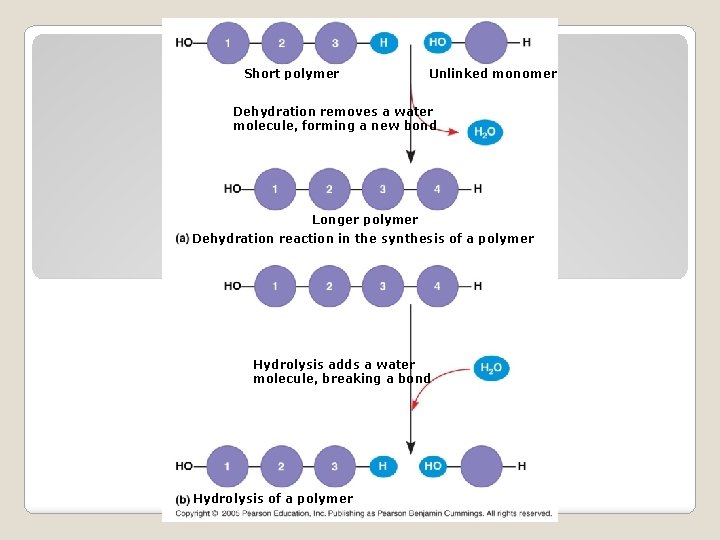
Short polymer Unlinked monomer Dehydration removes a water molecule, forming a new bond Longer polymer Dehydration reaction in the synthesis of a polymer Hydrolysis adds a water molecule, breaking a bond Hydrolysis of a polymer
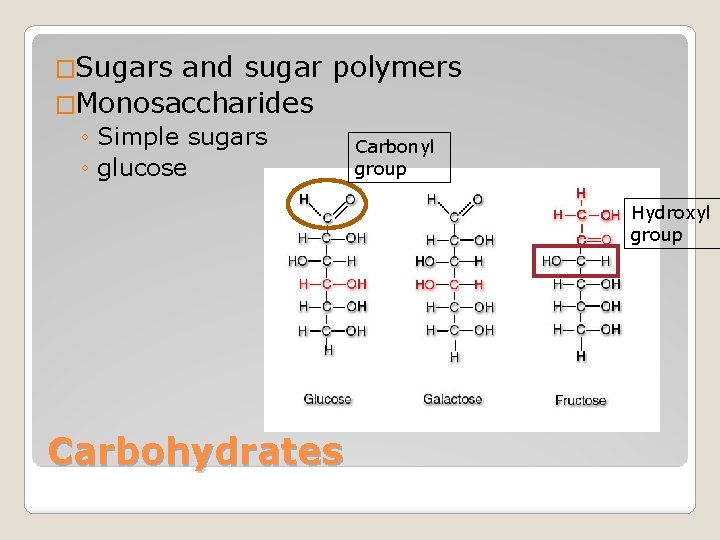
�Sugars and sugar polymers �Monosaccharides ◦ Simple sugars ◦ glucose Carbonyl group Hydroxyl group Carbohydrates
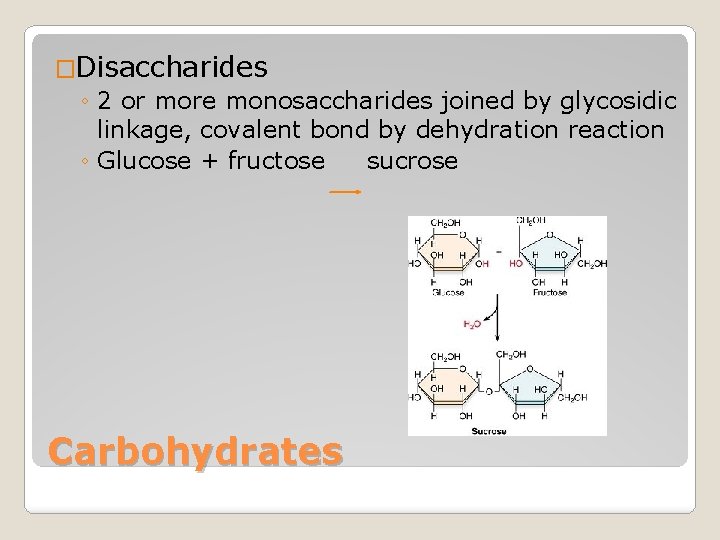
�Disaccharides ◦ 2 or more monosaccharides joined by glycosidic linkage, covalent bond by dehydration reaction ◦ Glucose + fructose sucrose Carbohydrates

�Storage ◦ Plant starch ◦ Stored energy can be broken down by hydrolysis into glucose ◦ Animal polysaccharide �Glycogen ◦ Stored in liver and muscles ◦ Used for short term energy Carbohydrates
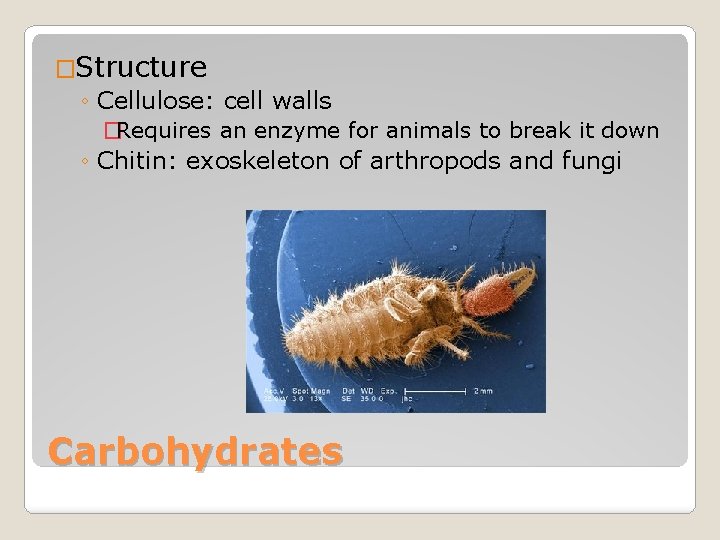
�Structure ◦ Cellulose: cell walls �Requires an enzyme for animals to break it down ◦ Chitin: exoskeleton of arthropods and fungi Carbohydrates
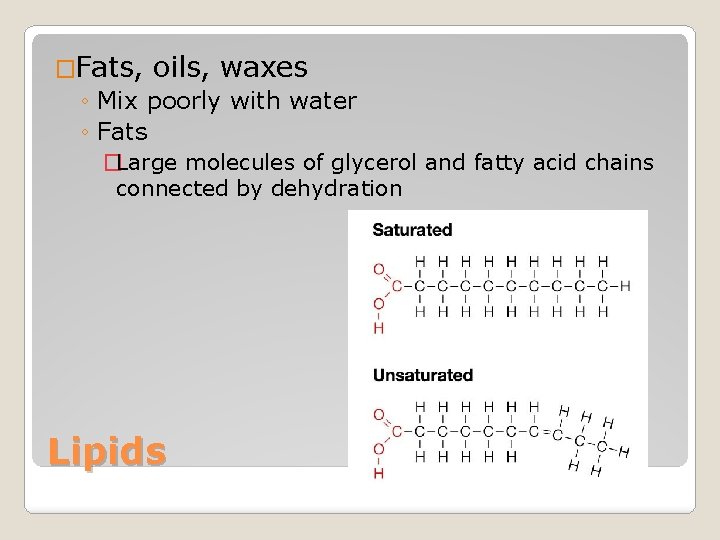
�Fats, oils, waxes ◦ Mix poorly with water ◦ Fats �Large molecules of glycerol and fatty acid chains connected by dehydration Lipids
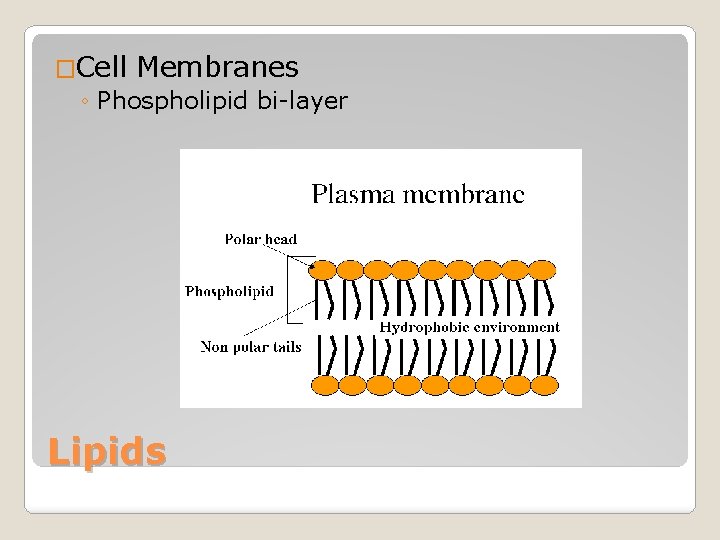
�Cell Membranes ◦ Phospholipid bi-layer Lipids

�Polymer of amino acids called polypeptides �Functions ◦ ◦ ◦ ◦ Enzymes Storage of amino acids Hormones Motor Defense Transport Receptors for chemical stimuli structure Proteins
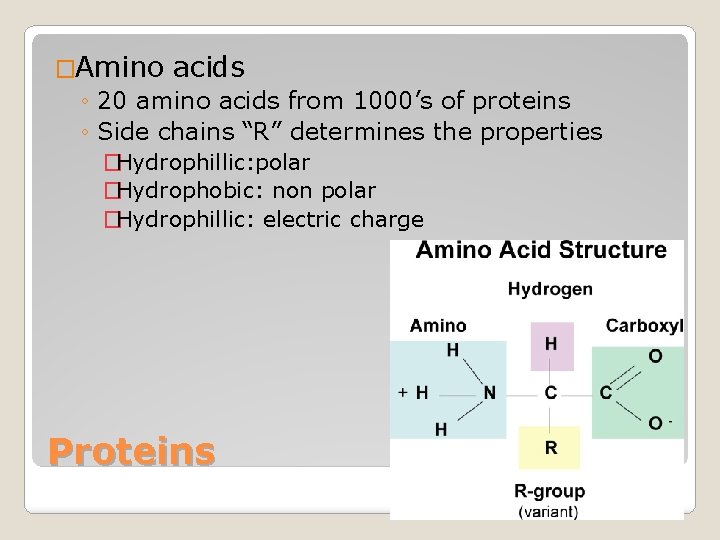
�Amino acids ◦ 20 amino acids from 1000’s of proteins ◦ Side chains “R” determines the properties �Hydrophillic: polar �Hydrophobic: non polar �Hydrophillic: electric charge Proteins
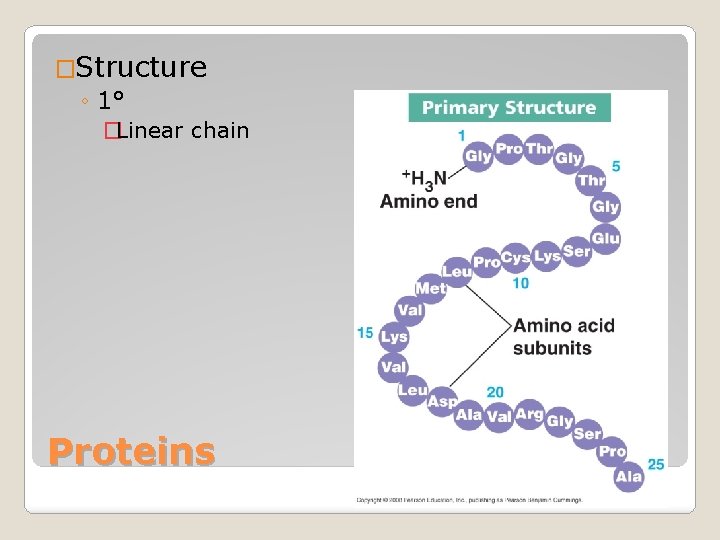
�Structure ◦ 1° �Linear chain Proteins
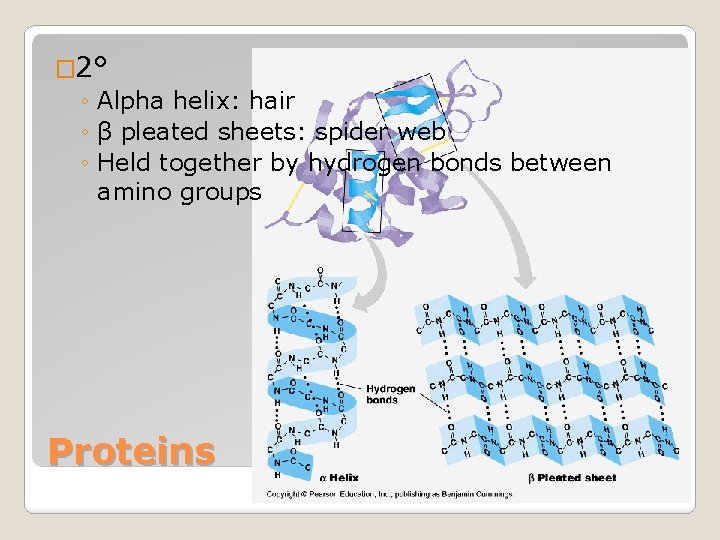
� 2° ◦ Alpha helix: hair ◦ β pleated sheets: spider web ◦ Held together by hydrogen bonds between amino groups Proteins
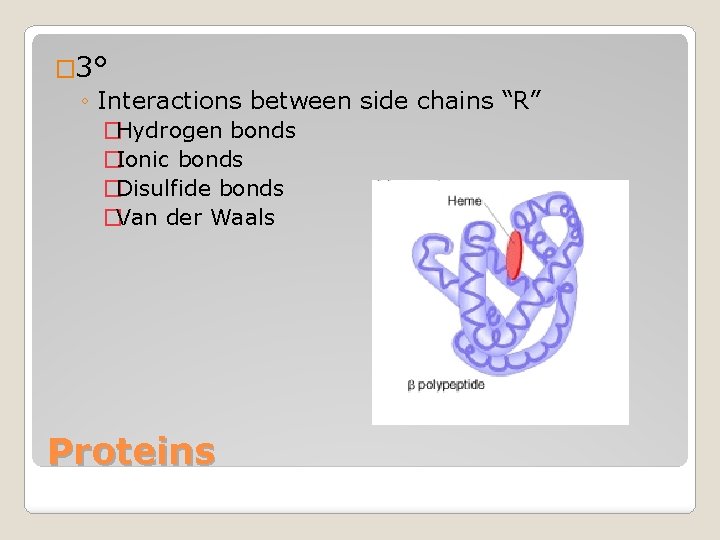
� 3° ◦ Interactions between side chains “R” �Hydrogen bonds �Ionic bonds �Disulfide bonds �Van der Waals Proteins
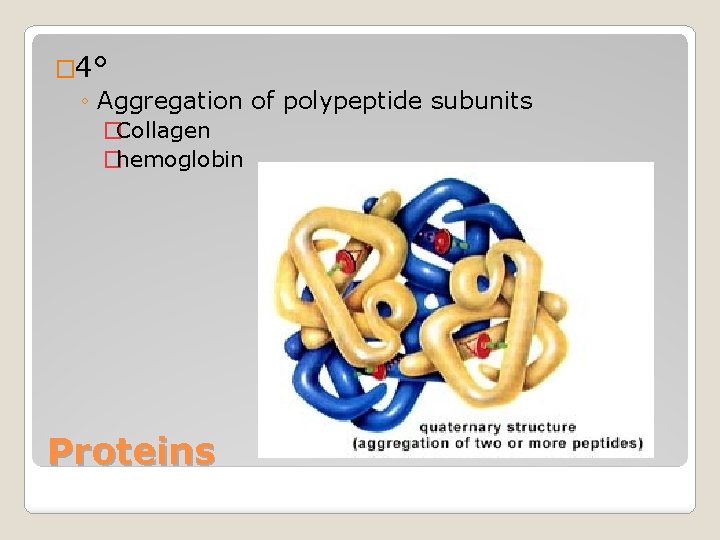
� 4° ◦ Aggregation of polypeptide subunits �Collagen �hemoglobin Proteins

�Denaturation ◦ Weak chemical bonds and interactions can be destroyed �Heat �p. H Proteins
�Polymer of nucleotides ◦ DNA and RNA Nucleic Acids
- Slides: 22