BUILDING BETTER BATTERIES WITH SOLID POLYMER ELECTROLYTE By

BUILDING BETTER BATTERIES WITH SOLID POLYMER ELECTROLYTE By A. Jagadeesan
Inside Introduction to Battery Different Types of Battery Lithium battery Li-ion battery principle, construction and working Advantage, disadvantage and applications My research work
How do we make and store electricity Today, we can convert energy from many different forms into usable electricity: • Coal • Wood • Nuclear fission • Oil • Water • Solar • Wind But the main problem for electricity is how we store and transport it from the generation source • Transmission wires • Batteries

Batteries definition A battery is a chemical device used for the generation or storage of electricity. Two or more electrochemical cells, electrically interconnected, each of which contains two electrodes and an electrolyte. The redox (oxidation-reduction) reactions that occur at these electrodes convert electrochemical energy into electrical energy. In 1800, Alessandro Volta invented the first modern battery.
Different Types of batteries Basically batteries can be classifieds as two types as primary batteries and secondary batteries. Primary batteries In primary batteries, the electrochemical reaction is not reversible. During discharging the chemical compounds are permanently changed and electrical energy is released until the original compounds are completely exhausted. Thus the cells can be used only once.
Secondary batteries • In secondary batteries, the electrochemical reaction is reversible and the original chemical compounds can be reconstituted by the application of an electrical potential between the electrodes injecting energy into the cell. • Such cells can be discharged and recharged many times.
There are two types of lithium-based batteries available. 1. Lithium batteries 2. Lithium-ion batteries • In lithium batteries, a pure lithium metallic element is used as anode. These types of batteries are not rechargeable. • In lithium-ion batteries, lithium compounds are used as anode. • These batteries are known as re-chargeable batteries. Therefore, Lithium ion batteries are considered as best than pure Lithium based batteries.

Lithium battery • Lithium is the lightest of metals and it can float on water. • The electrochemical properties of lithium are excellent and it is also a highly reactive material. • These properties gives Lithium the potential to achieve very high energy and power densities in high-density battery applications such as automotive and standby power. • Lithium batteries are primary batteries in which lithium metal (or) lithium compound acts as a Anode. A lithium cell can produce voltage from 1. 5 V to about 3 V based on the types of materials used.

Lithium-ion battery (Li-ion Battery) Li-ion batteries are secondary batteries. • The battery consists of a anode of Lithium, dissolved as ions, into a carbon. • The cathode material is made up from Lithium liberating compounds, typically the three electro-active oxide materials, • • • Lithium Cobalt-oxide (Li. Co. O 2 ) Lithium Manganese-oxide (Li. Mn 2 O 4 ) Lithium Nickel-oxide (Li. Ni. O 2)
Principle • ions are inserted or extracted from interstitial space between atomic layers within the active material of the battery. • cathode through lithium Electrolyte. • essentially change, the operation is safer than that of a Lithium metal battery.
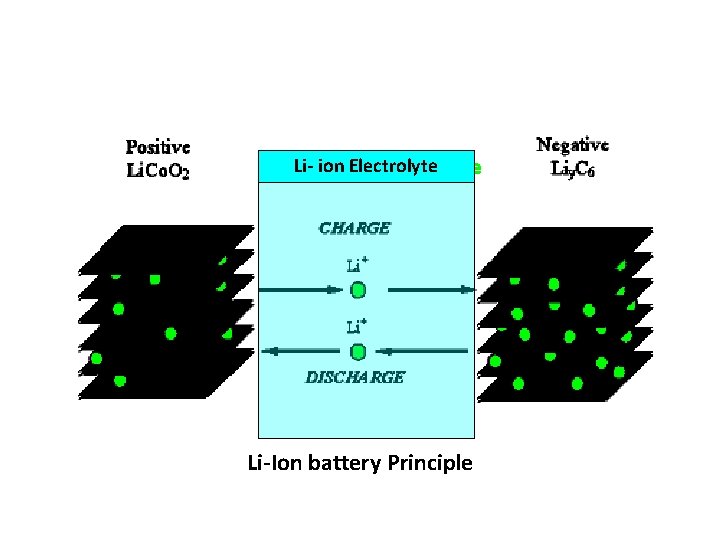
Li- ion Electrolyte Li-Ion battery Principle
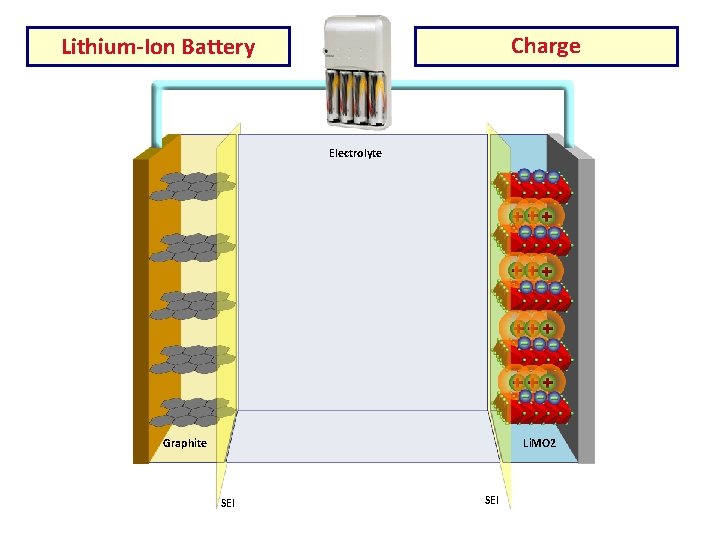
Charge Lithium-Ion Battery Electrolyte Cu Current AL Current Collector Graphite Li. MO 2 SEI
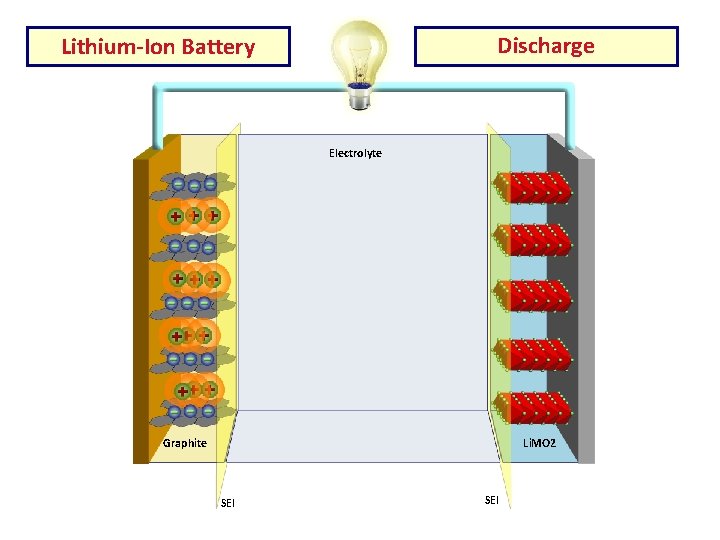
Discharge Lithium-Ion Battery Electrolyte Cu Current AL Current Collector Graphite Li. MO 2 SEI

Construction • Li-ion cell has a four-layer structure. • has a current collector made of thin aluminum foil - cathode • current collector of thin copper foil – anode • A separator is a fine porous polymer film. • An electrolyte made with lithium salt in an organic solvent.

Working The traditional batteries are based on galvanic action but Lithium ion secondary battery depends on an "intercalation" mechanism. This involves the insertion of lithium ions into the crystalline lattice of the host electrode without changing its crystal structure. These electrodes have two key properties. One is the open crystal structure, which allow the insertion or extraction of lithium ions and the second is the ability to accept compensating electrons at the same time. Such electrodes are called intercalation hosts.
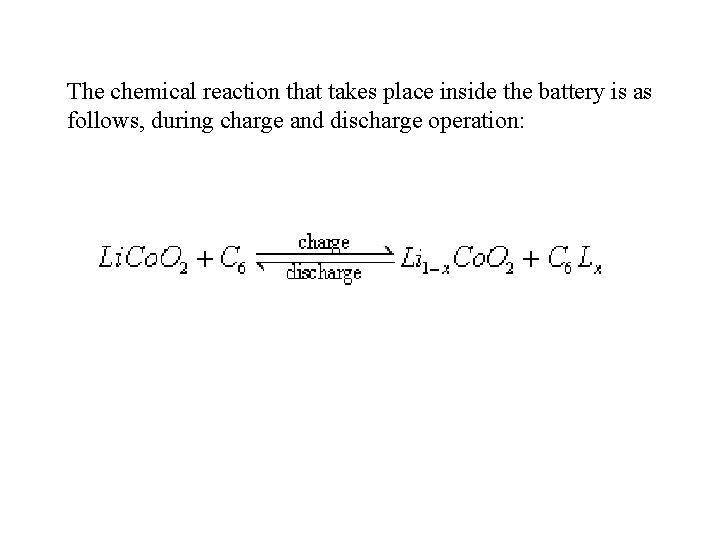
The chemical reaction that takes place inside the battery is as follows, during charge and discharge operation:
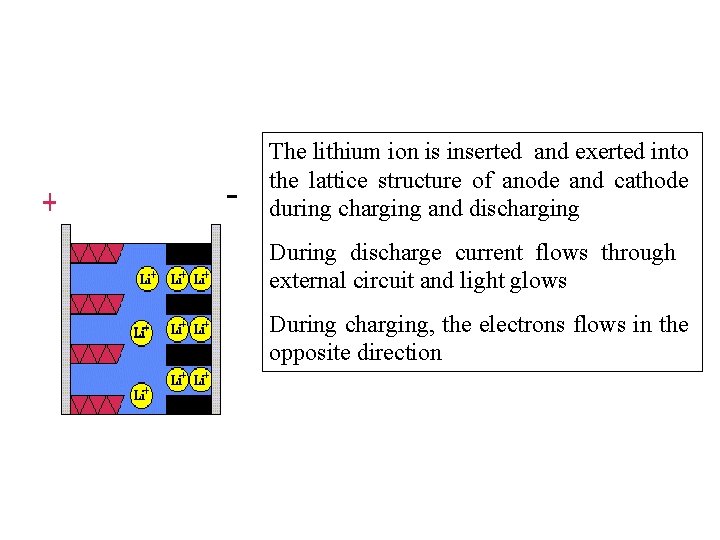
The lithium ion is inserted and exerted into the lattice structure of anode and cathode during charging and discharging During discharge current flows through external circuit and light glows During charging, the electrons flows in the opposite direction
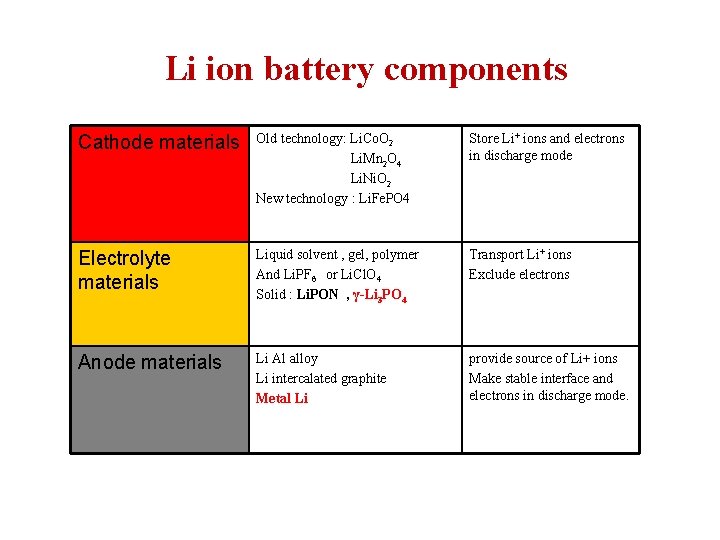
Li ion battery components Cathode materials Old technology: Li. Co. O 2 Li. Mn 2 O 4 Li. Ni. O 2 New technology : Li. Fe. PO 4 Store Li+ ions and electrons in discharge mode Electrolyte materials Liquid solvent , gel, polymer And Li. PF 6 or Li. Cl. O 4 Solid : Li. PON , γ-Li 3 PO 4 Transport Li+ ions Exclude electrons Anode materials Li Al alloy Li intercalated graphite Metal Li provide source of Li+ ions Make stable interface and electrons in discharge mode.

Key Battery Attributes • Energy Density: Total amount of energy that can be stored per unit mass or volume. How long will your laptop run before it must be recharged? • Power Density: Maximum rate of energy discharge per unit mass or volume. Low power: laptop, i-pod. High power: power tools. • Safety: At high temperatures, certain battery components will breakdown and can undergo exothermic reactions. • Life: Stability of energy density and power density with repeated cycling is needed for the long life required in many applications. • Cost: Must compete with other energy storage technologies.
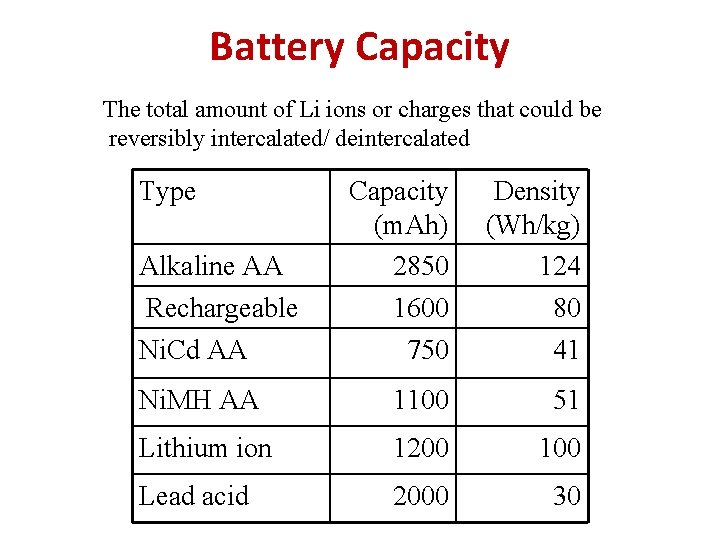
Battery Capacity The total amount of Li ions or charges that could be reversibly intercalated/ deintercalated Type Capacity (m. Ah) 2850 1600 Density (Wh/kg) 124 80 Ni. Cd AA 750 41 Ni. MH AA 1100 51 Lithium ion 1200 100 Lead acid 2000 30 Alkaline AA Rechargeable
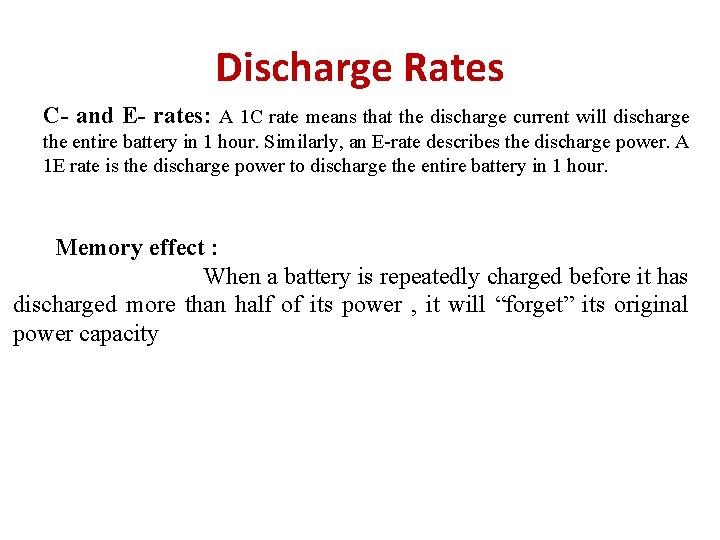
Discharge Rates C- and E- rates: A 1 C rate means that the discharge current will discharge the entire battery in 1 hour. Similarly, an E-rate describes the discharge power. A 1 E rate is the discharge power to discharge the entire battery in 1 hour. Memory effect : When a battery is repeatedly charged before it has discharged more than half of its power , it will “forget” its original power capacity
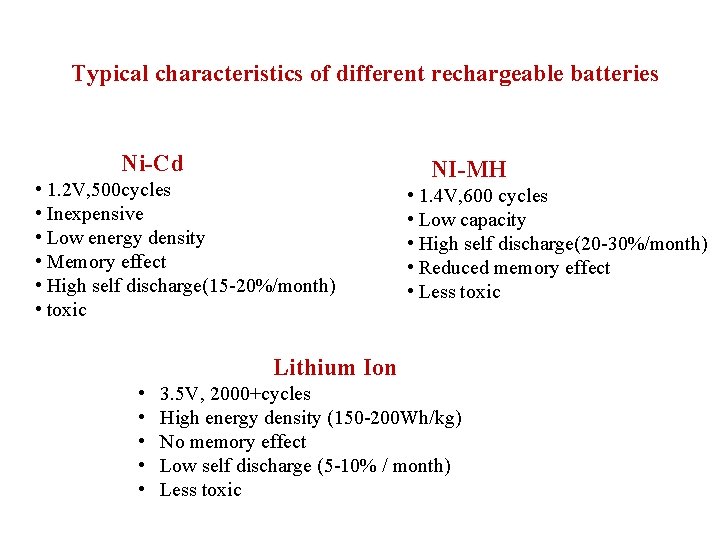
Typical characteristics of different rechargeable batteries Ni-Cd • 1. 2 V, 500 cycles • Inexpensive • Low energy density • Memory effect • High self discharge(15 -20%/month) • toxic NI-MH • 1. 4 V, 600 cycles • Low capacity • High self discharge(20 -30%/month) • Reduced memory effect • Less toxic Lithium Ion • • • 3. 5 V, 2000+cycles High energy density (150 -200 Wh/kg) No memory effect Low self discharge (5 -10% / month) Less toxic

Cathode Materials Challenges • The most desirable cathode materials are strong oxidizing agents that can react with and decompose organic electrolytes • In extreme cases, problems with internal shorts or improper voltages can trigger exothermic reactions, leading to thermal runaway and catastrophic failure
Electrolyte Challenges: Liquid electrolyte • Problems : leakage, sealing, non-flexibility of the cells, side reactions with charged electrodes; ↓ Explosions
None of the existing electrode materials alone can deliver all the required performance characteristics including high capacity, higher operating voltage, long cycle life and safety. ↓ RESEARCH AND DEVELOPMENT
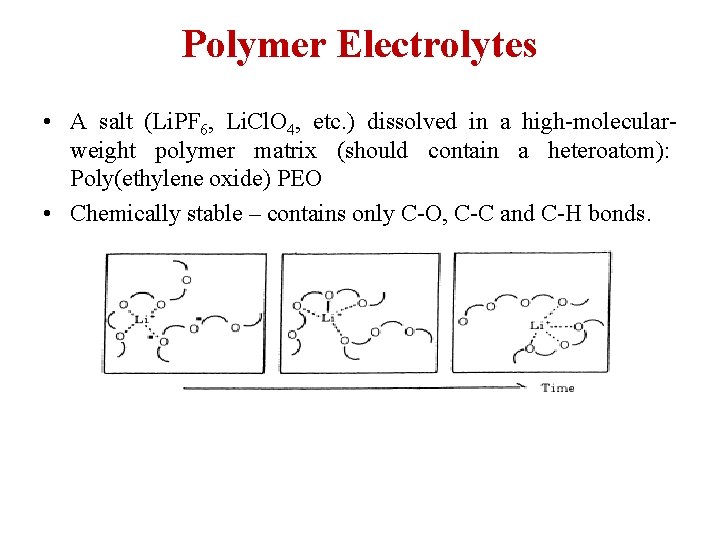
Polymer Electrolytes • A salt (Li. PF 6, Li. Cl. O 4, etc. ) dissolved in a high-molecularweight polymer matrix (should contain a heteroatom): Poly(ethylene oxide) PEO • Chemically stable – contains only C-O, C-C and C-H bonds.
Solid polymer electrolytes: advantages to liquid electrolytes • High reversibility of the processes (high electrochemical stability); • Solid => no risk of leakage of electrolyte; • Can be used in a wider range of temperature; • Light weight • High Flexibility • Possibility of miniaturization.
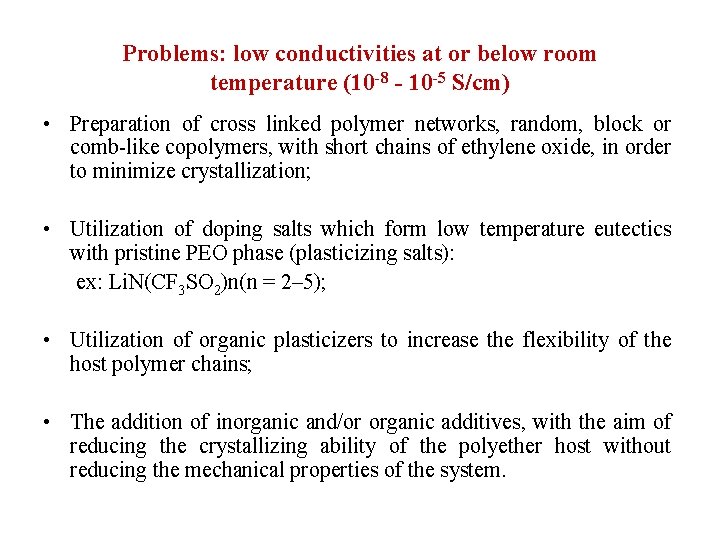
Problems: low conductivities at or below room temperature (10 -8 - 10 -5 S/cm) • Preparation of cross linked polymer networks, random, block or comb-like copolymers, with short chains of ethylene oxide, in order to minimize crystallization; • Utilization of doping salts which form low temperature eutectics with pristine PEO phase (plasticizing salts): ex: Li. N(CF 3 SO 2)n(n = 2– 5); • Utilization of organic plasticizers to increase the flexibility of the host polymer chains; • The addition of inorganic and/or organic additives, with the aim of reducing the crystallizing ability of the polyether host without reducing the mechanical properties of the system.
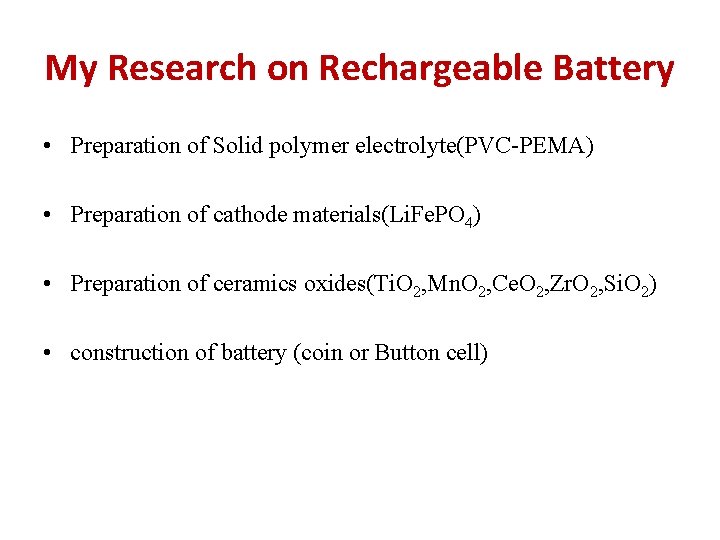
My Research on Rechargeable Battery • Preparation of Solid polymer electrolyte(PVC-PEMA) • Preparation of cathode materials(Li. Fe. PO 4) • Preparation of ceramics oxides(Ti. O 2, Mn. O 2, Ce. O 2, Zr. O 2, Si. O 2) • construction of battery (coin or Button cell)
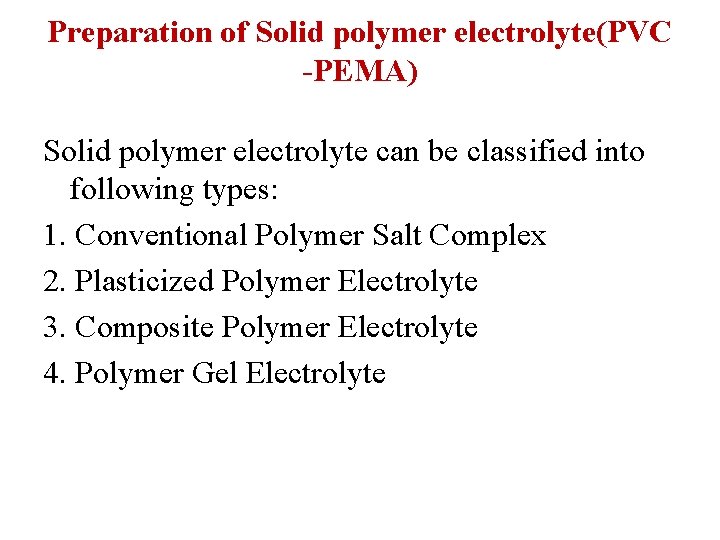
Preparation of Solid polymer electrolyte(PVC -PEMA) Solid polymer electrolyte can be classified into following types: 1. Conventional Polymer Salt Complex 2. Plasticized Polymer Electrolyte 3. Composite Polymer Electrolyte 4. Polymer Gel Electrolyte

Conventional Polymer Salt Complex • Dry solid polymer electrolyte can be prepared by complexation of a salt in a polar polymer host like PPO, PEO etc. and also known as polymer – salt complex. • The observed ionic conductivity of this polymer–salt complex is very low as compared to the desired value.

Plasticized Polymer Electrolyte • It can be prepared by adding a plasticizer, which is a low molecular weight and high dielectric constant material to the conventional polymer salt complex. • The introduction of a plasticizer significantly improves the ionic conductivity as well as flexibility to the system, but the mechanical strength decreases. Still the observed ionic conductivity is below the desired level.

Polymer Gel Electrolyte • Polymer gel electrolyte can be prepared by adding non-conducting polymer to a liquid electrolyte in a balanced ratio so that polymerization will produce a gel. • The polymer is added to give the mechanical support to the system. So at macroscopic level it behaves like a solid and liquid like properties is observed at microscopic level.
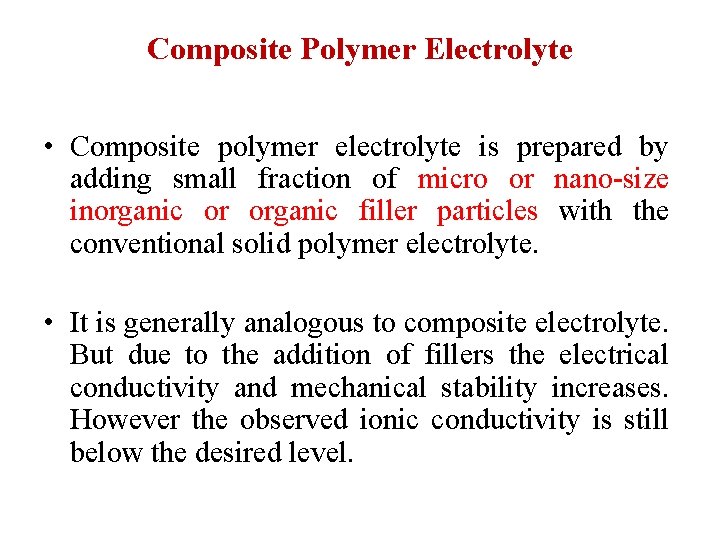
Composite Polymer Electrolyte • Composite polymer electrolyte is prepared by adding small fraction of micro or nano-size inorganic or organic filler particles with the conventional solid polymer electrolyte. • It is generally analogous to composite electrolyte. But due to the addition of fillers the electrical conductivity and mechanical stability increases. However the observed ionic conductivity is still below the desired level.
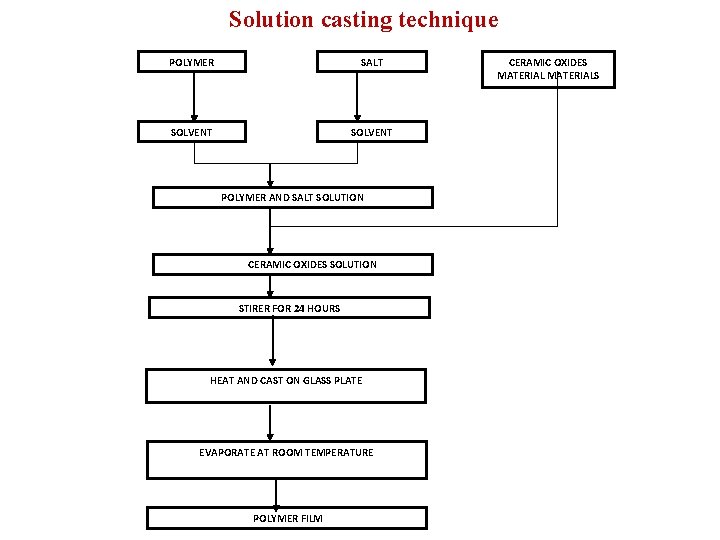
Solution casting technique POLYMER SALT SOLVENT POLYMER AND SALT SOLUTION CERAMIC OXIDES SOLUTION STIRER FOR 24 HOURS HEAT AND CAST ON GLASS PLATE EVAPORATE AT ROOM TEMPERATURE POLYMER FILM CERAMIC OXIDES MATERIALS
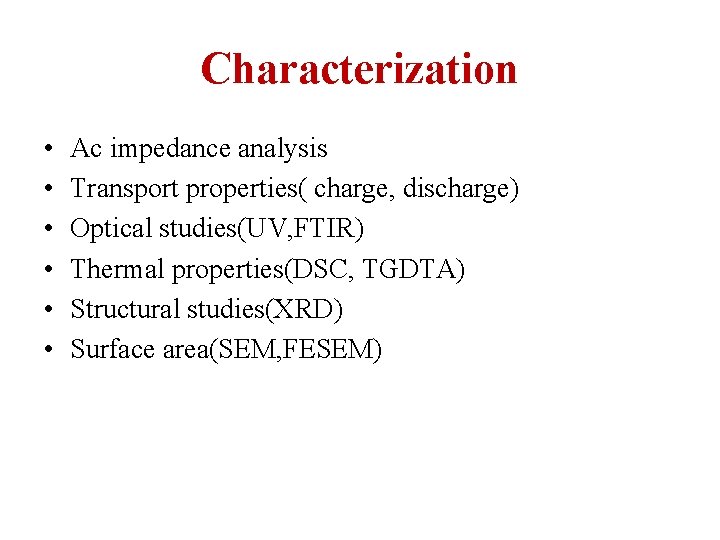
Characterization • • • Ac impedance analysis Transport properties( charge, discharge) Optical studies(UV, FTIR) Thermal properties(DSC, TGDTA) Structural studies(XRD) Surface area(SEM, FESEM)
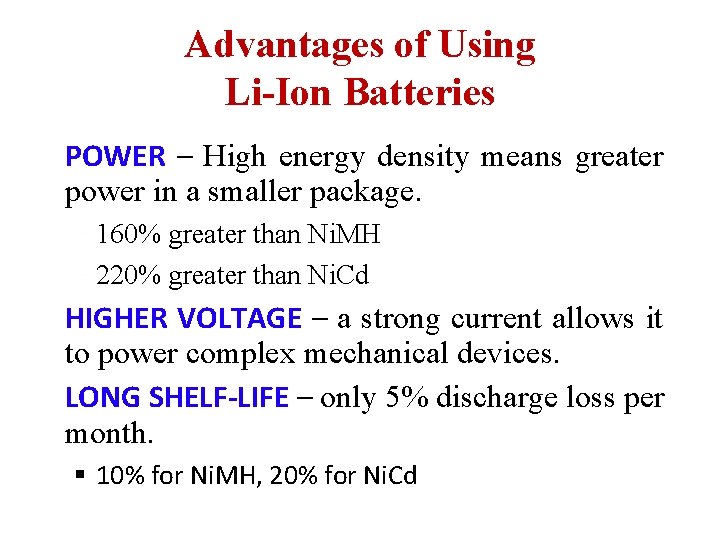
Advantages of Using Li-Ion Batteries POWER – High energy density means greater power in a smaller package. 160% greater than Ni. MH 220% greater than Ni. Cd HIGHER VOLTAGE – a strong current allows it to power complex mechanical devices. LONG SHELF-LIFE – only 5% discharge loss per month. § 10% for Ni. MH, 20% for Ni. Cd

Disadvantages of Li-Ion EXPENSIVE -- 40% more than Ni. Cd. DELICATE -- battery temp must be monitored from within (which raises the price), and sealed particularly well. REGULATIONS -- when shipping Li-Ion batteries in bulk (which also raises the price).

Environmental Impact of Li-Ion Batteries Rechargeable batteries are often recyclable. Oxidized Lithium is non-toxic, and can be extracted from the battery, neutralized, and used as feedstock for new Li-Ion batteries.
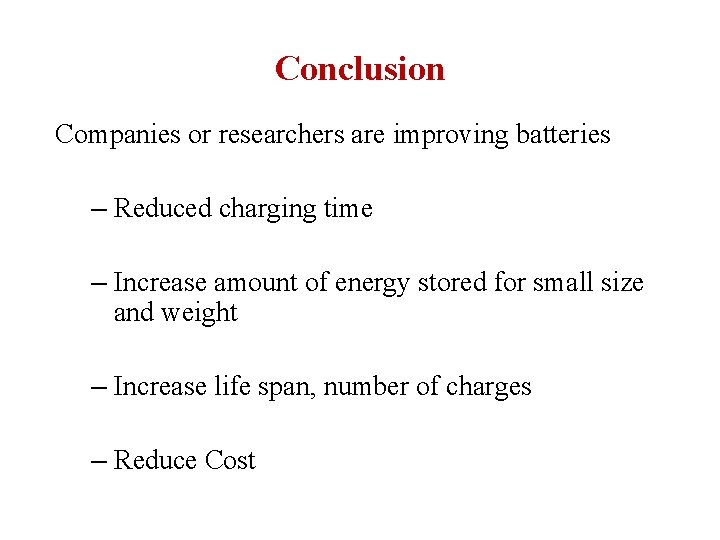
Conclusion Companies or researchers are improving batteries – Reduced charging time – Increase amount of energy stored for small size and weight – Increase life span, number of charges – Reduce Cost

Thank you for your attention
- Slides: 42