Building and Maintaining a Sound Clinical Research Program

Building and Maintaining a Sound Clinical Research Program Alexandra Lansky, MD Associate Professor of Medicine Yale University School of Medicine Honorary Reader, University College London

No Conflicts
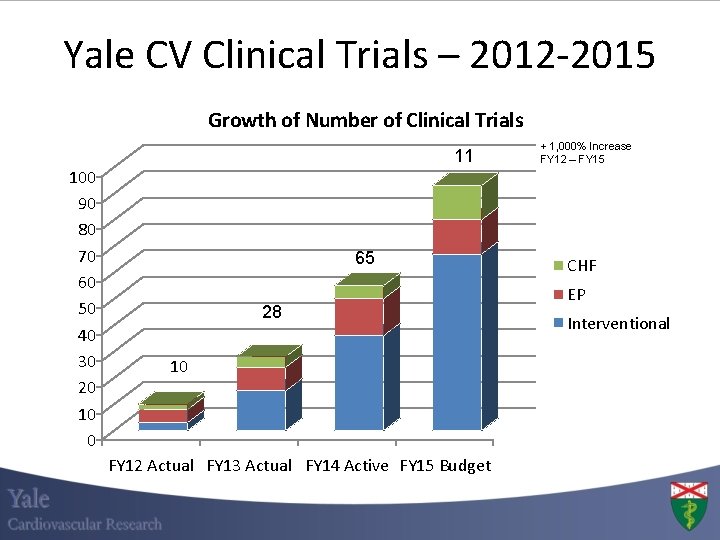
Yale CV Clinical Trials – 2012 -2015 Growth of Number of Clinical Trials 100 90 80 70 60 50 40 30 20 10 0 11 65 28 10 FY 12 Actual FY 13 Actual FY 14 Active FY 15 Budget + 1, 000% Increase FY 12 – FY 15 CHF EP Interventional

Keys to Success • A committed Team • Strong collaboration • Strong leadership • Strong partnership • Strong expertise • Financial viability • A research culture

Why do we do this? • Improve patient care • Leadership in the field • Academic kudos • Access to new technology • Hospital ranking • Increase volume • It’s FUN!!!!
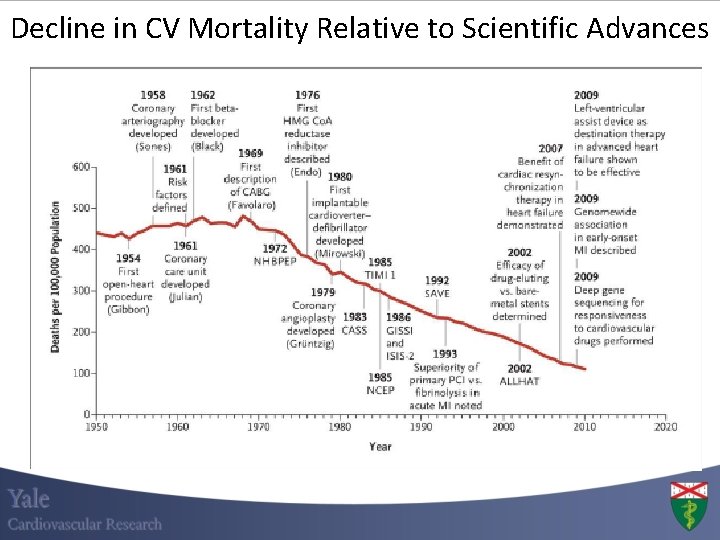
Decline in CV Mortality Relative to Scientific Advances
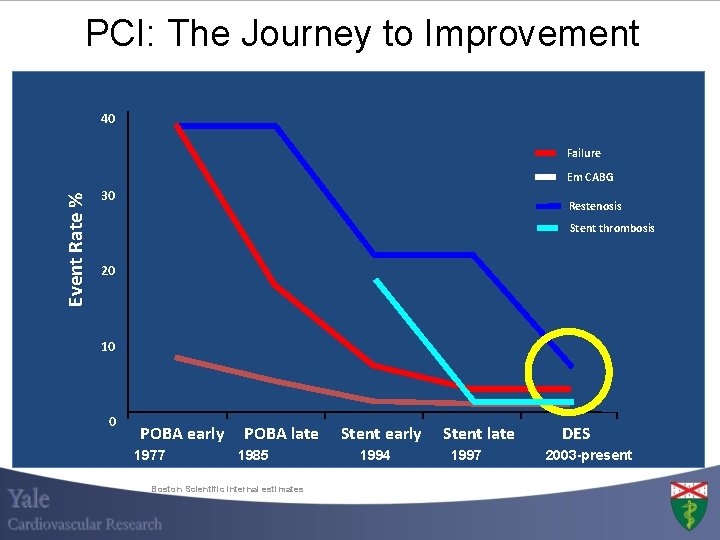
PCI: The Journey to Improvement 40 Failure Event Rate % Em CABG 30 Restenosis Stent thrombosis 20 10 0 POBA early 1977 POBA late 1985 Boston Scientific internal estimates Stent early 1994 Stent late 1997 DES 2003 -present
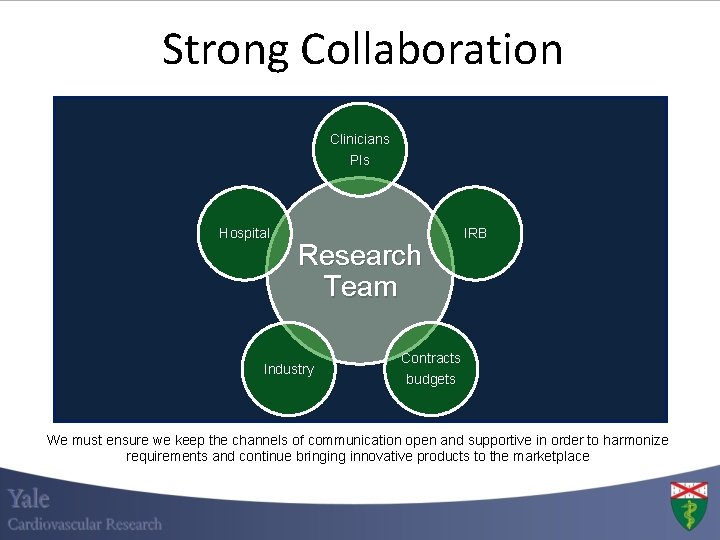
Strong Collaboration Clinicians PIs Hospital Research Team Industry IRB Contracts budgets We must ensure we keep the channels of communication open and supportive in order to harmonize requirements and continue bringing innovative products to the marketplace

PI has to Champion the program • A research culture • Invested in success • A strong leader • Accessible • Passionate • Proactive • Wants to enroll • Good communicator
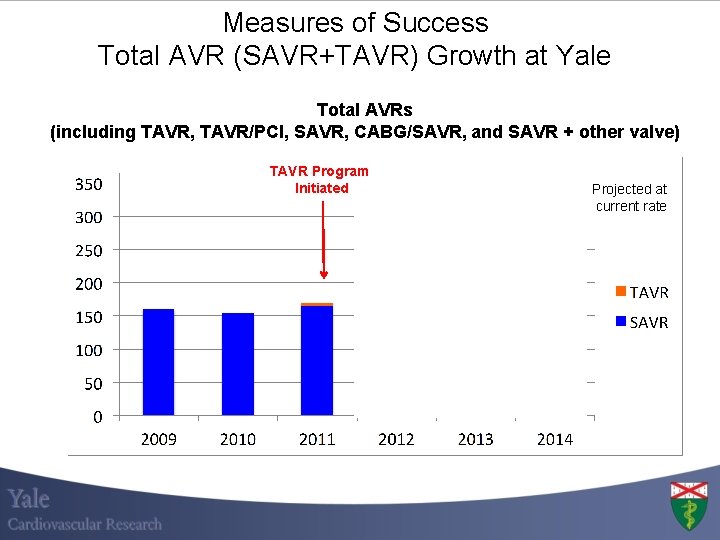
Measures of Success Total AVR (SAVR+TAVR) Growth at Yale Total AVRs (including TAVR, TAVR/PCI, SAVR, CABG/SAVR, and SAVR + other valve) TAVR Program Initiated * Projected at current rate

Research Coordinator makes or breaks the program • Enthusiastic • Owns the trial • Loves patients • Feels empowered • Good communicator • Nurturing environment • Good perks: investigator meetings • Good training

Introducing the Amazing: Study Coordinator Doll “Do I have to report this to the IRB? ” She Walks! She Talks! Never needs batteries! Runs on Coffee and Chocolate! Works 60 -80 hours a week! (on an incredible 0. 5 She Works Without Breaks! FTE)! (Just like a REAL CRC!) Sign here, and Sign here and here Fun Accessories: - cell phone - lap top - instant resource - investigator and patient tracker

Don’t get bogged down at the outset Clear the IRB Sign the Contracts

Choose your protocols selectively • Right population • Right expertise • Interest • Right equipment • Feasible • Financially viable
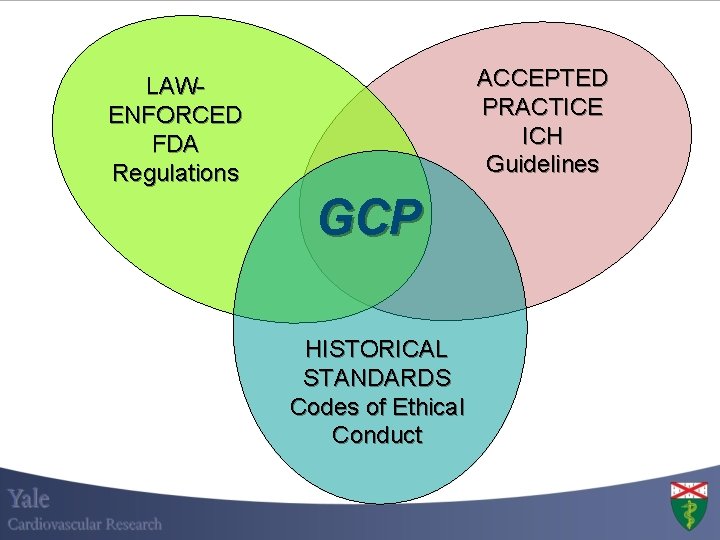
ACCEPTED PRACTICE ICH Guidelines LAWENFORCED FDA Regulations GCP HISTORICAL STANDARDS Codes of Ethical Conduct

Financial Viability… as for any successful business • Dedicated Financial manager • Budgets • Invoicing • Internal reconciliation • Projections • Financial modeling

At the end of the day Its all about the patient….

- Slides: 18