Building a National Medication Reference Terminology VA Experience

Building a National Medication Reference Terminology: VA Experience with NDF-RT Presented to HL 7 Government SIG By Steven Brown & Michael Lincoln, Department of Veterans Affairs In collaboration with NLM, FDA, NCI and Apelon

Why a Common Drug Reference Terminology? • Improve use of drug information by single systems • Improve sharing of drug information by adding semantic understanding to the syntax defined by HL 7 messaging between systems 2

VA National Drug File (NDF) • • • Centrally maintained file of drug products Distributed to 128 VA Medical Centers Incorporated into Vist. A Pharmacy Applications ~ 80, 000 Entries Maintained and used via “M Globals” – VA Product File – VA Active Ingredient File • More than a decade of experience… 3

NDF Uses Within VA • • National standard listing Local formularies mapped to NDF standard Interaction and Order Checking Consolidated Mail Outpatient Pharmacy – > 7 sites, 57 million prescriptions/year 4

NDF Product File • Product Names – AMOXICILLIN 250 MG/CLAVULANATE K 125 MG TAB • Form, Strength, Product Identifier • Linked to: VA Drug Class : – AM 052 “penicillins, amino derivatives” • Linked to: Active Ingredients 5

NDF - Issues • Maintenance – 4000 NDC level edits per month – Done largely by hand • Mapping local formularies to NDF – Manual process – Required for CMOP • Decision Support – class based – CN 101 “Opioid Analgesics” vs. CN 103 “Non-opioid analgesics” 6

NDF Reference Terminology (RT) • Enterprise Architecture project • Goal – to evaluate the use of modern terminology techniques to increase functionality, improve quality and decrease costs 7

Rossi Mori First Generation Terminology • Traditional Paper-Based Terminological Systems with lists of phrases and codes. – E. g. ICD 9, CPT 8

Rossi Mori Second Generation Terminology • Second Generation Terminology – Compositional terminological systems built according to a categorical structure and a crossthesaurus with predefined values for each category. – E. g. LOINC 9

Rossi Mori Third Generation Terminology • Reference Terminology – – A terminology where each term has a formal definition designed for data aggregation and retrieval. – Formal terminological systems represent concepts with symbols and rules that create a computable structured and coded system – e. g. SNOMED RT, NDF RT 10
Advantages of Formal Terminology • Computer-based tools – Reduced Maintenance Costs • 90% reduction in MED Maintenance effort – Reduced Mapping Effort • 60% automated matches to formalized version of LOINC • Comparable Data • Supports Aggregation 11
Reference Terminology Creation • Create a model of use case-based definitional attributes • Create a reference hierarchy for each definitional attribute • Define concepts using terms from reference hierarchies 12
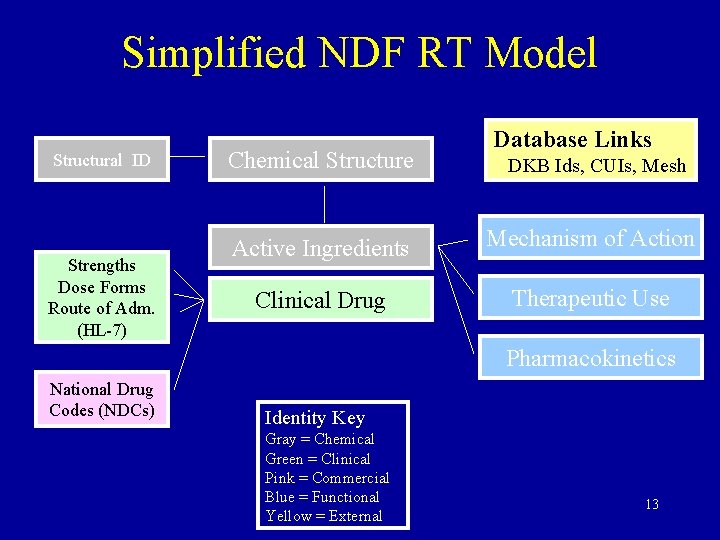
Simplified NDF RT Model Structural ID Strengths Dose Forms Route of Adm. (HL-7) Chemical Structure Database Links DKB Ids, CUIs, Mesh Active Ingredients Mechanism of Action Clinical Drug Therapeutic Use Pharmacokinetics National Drug Codes (NDCs) Identity Key Gray = Chemical Green = Clinical Pink = Commercial Blue = Functional Yellow = External 13
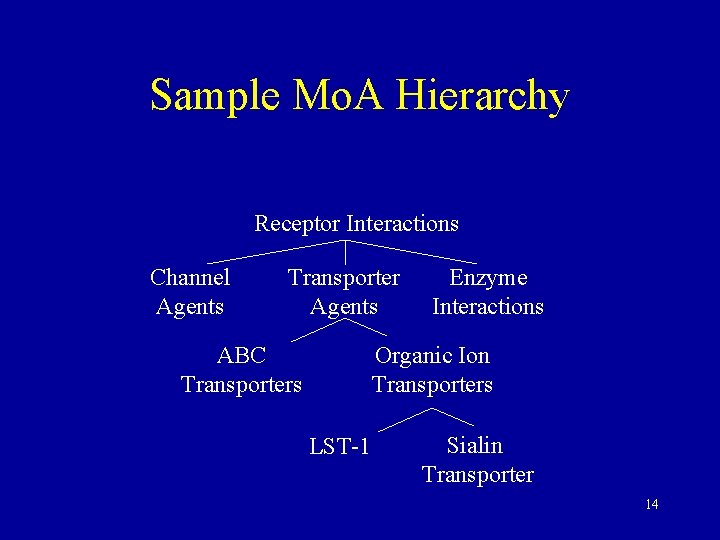
Sample Mo. A Hierarchy Receptor Interactions Channel Agents Transporter Agents ABC Transporters Enzyme Interactions Organic Ion Transporters LST-1 Sialin Transporter 14

Sample Definition AMOXICILLIN 250 MG/CLAVULANATE K 125 MG TAB – Has_form: tablet – Has_active_ingredient: Amoxicillin • Has_strength 250 mg • Has_Mo. A: cell wall synthesis disruption – Has_active_ingredient: Clavulanate • Has_strength 125 mg 15

NDF RT Progress to Date • NDF in terminology development tool • Model Created • Reference Taxonomies – Done: Route, Form, Strength, Structure – Under development : Mo. A, Physiologic Effect, Therapeutic Intent and Pharmacokinetics • Concept Definition – Algorithmic initialization – Human review • Input from NLM, HL 7, FDA, NCI, Apelon 16

NDF RT – Next Steps • Sample Applications demonstrating value • Expand Project beyond VA – NLM, FDA, NCI, Regenstrief, ? ? ? – Freely available to others for reuse 17
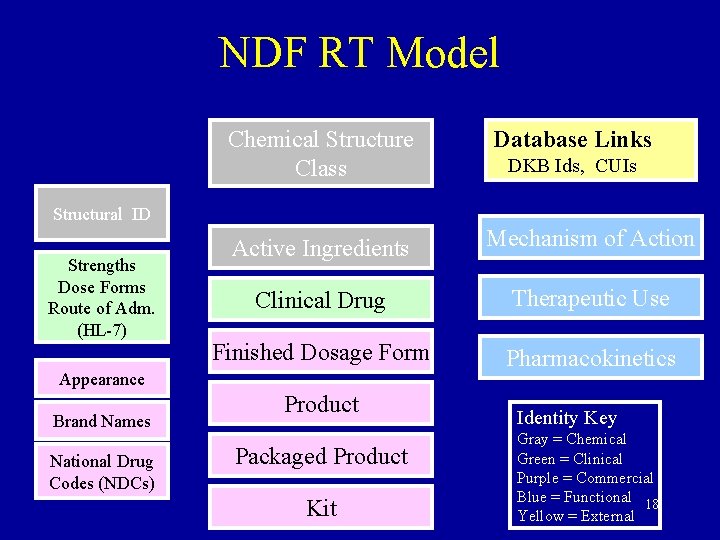
NDF RT Model Chemical Structure Class Database Links Active Ingredients Mechanism of Action Clinical Drug Therapeutic Use Finished Dosage Form Pharmacokinetics DKB Ids, CUIs Structural ID Strengths Dose Forms Route of Adm. (HL-7) Appearance Brand Names National Drug Codes (NDCs) Product Packaged Product Kit Identity Key Gray = Chemical Green = Clinical Purple = Commercial Blue = Functional 18 Yellow = External
- Slides: 18