Buffers and Titrations The Common Ion Effect Buffer

Buffers and Titrations
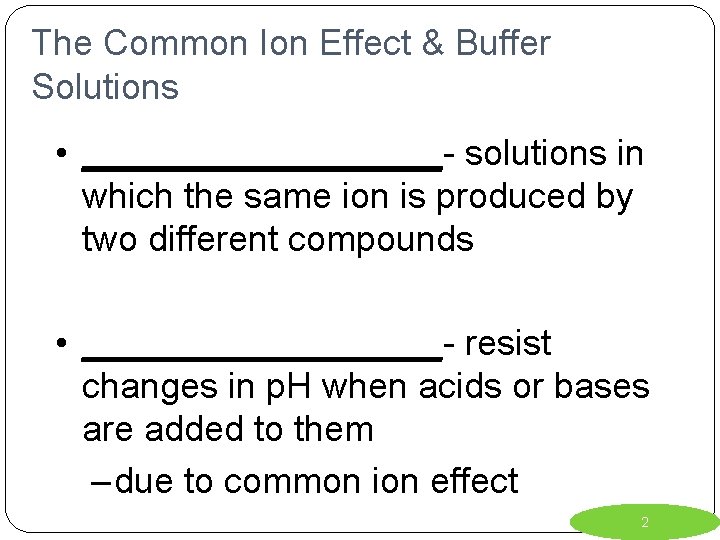
The Common Ion Effect & Buffer Solutions • _________- solutions in which the same ion is produced by two different compounds • _________- resist changes in p. H when acids or bases are added to them – due to common ion effect 2
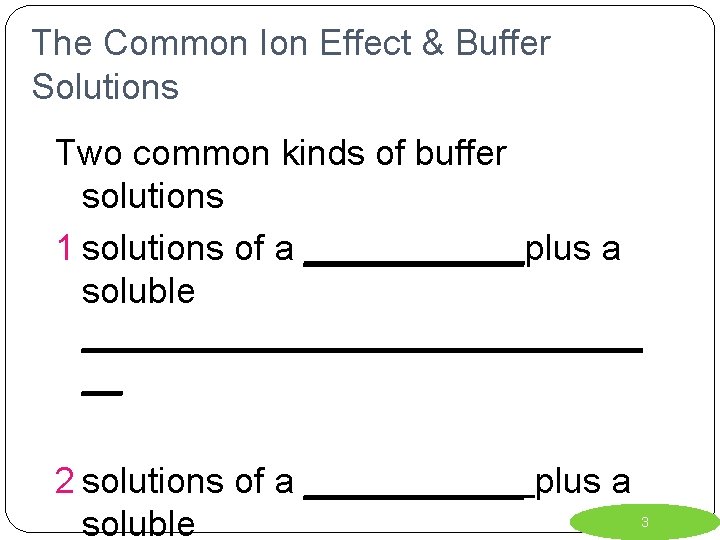
The Common Ion Effect & Buffer Solutions Two common kinds of buffer solutions 1 solutions of a ______plus a soluble ______________ __ 2 solutions of a ______ plus a soluble 3
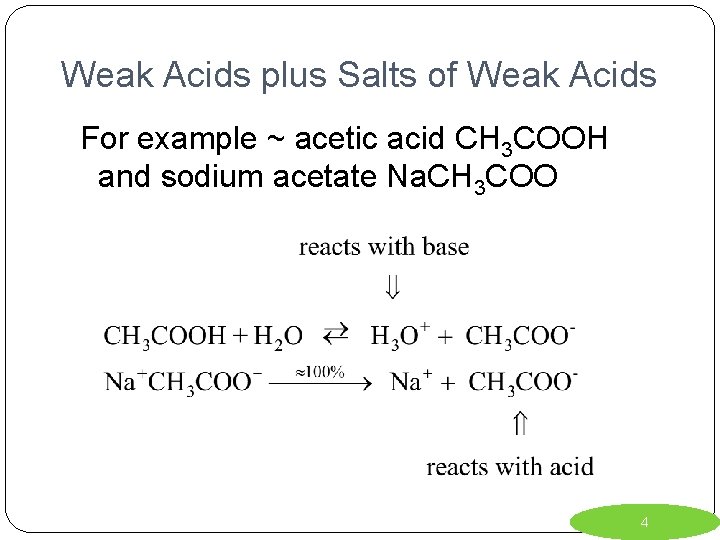
Weak Acids plus Salts of Weak Acids For example ~ acetic acid CH 3 COOH and sodium acetate Na. CH 3 COO 4

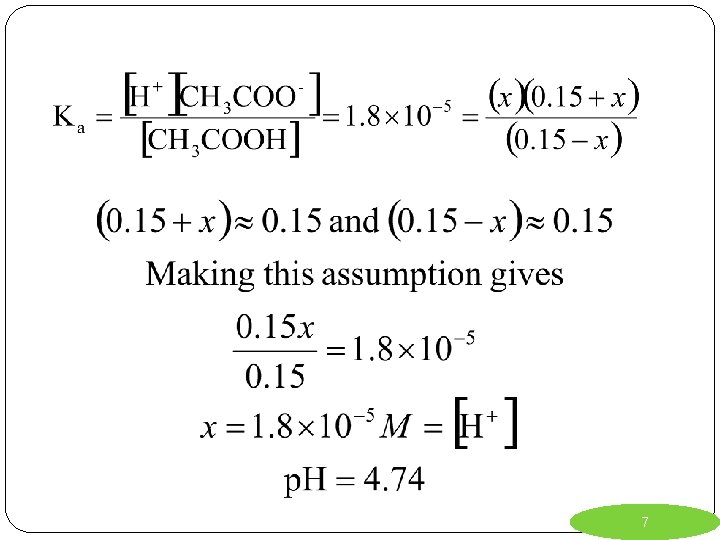
Ex. 1) Calculate the concentration of H+ and the p. H of a solution that is 0. 15 M in acetic acid and 0. 15 M in sodium acetate. Ka = 1. 8 x 10 -5 *Always start the reaction with your weak acid (or weak base) added to water. (note: sodium acetate completely dissociates) R I. C. E. 5
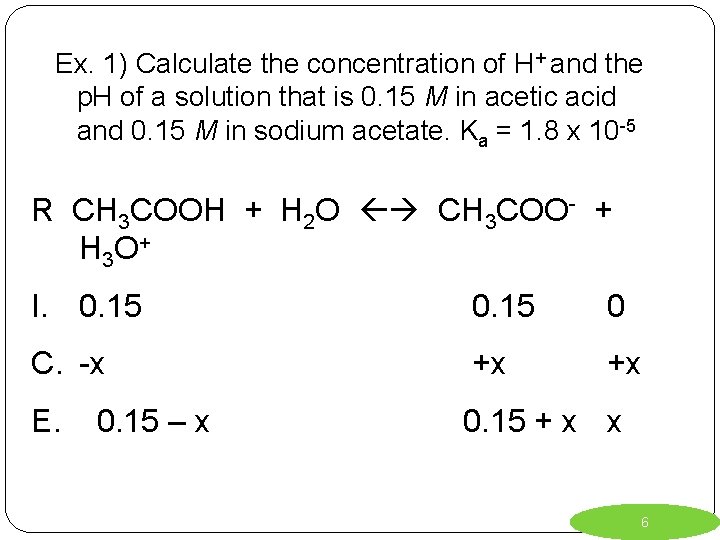
Ex. 1) Calculate the concentration of H+ and the p. H of a solution that is 0. 15 M in acetic acid and 0. 15 M in sodium acetate. Ka = 1. 8 x 10 -5 R CH 3 COOH + H 2 O CH 3 COO- + H 3 O + I. 0. 15 0 C. -x +x +x E. 0. 15 + x x 0. 15 – x 6
7
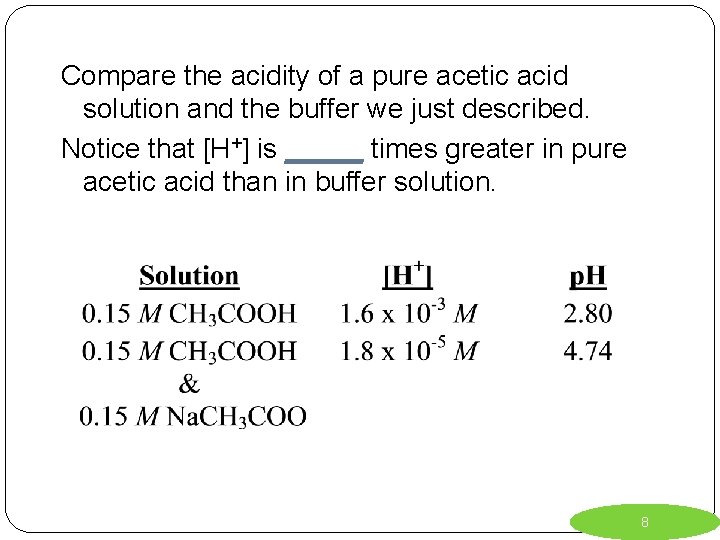
Compare the acidity of a pure acetic acid solution and the buffer we just described. Notice that [H+] is _____ times greater in pure acetic acid than in buffer solution. 8
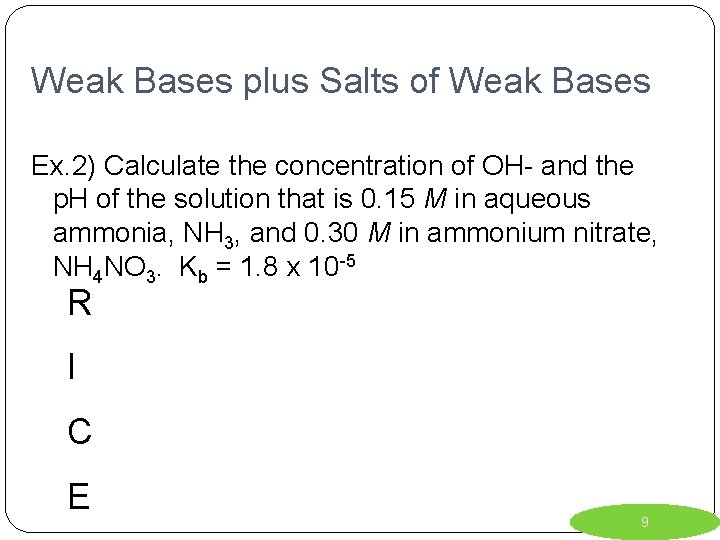
Weak Bases plus Salts of Weak Bases Ex. 2) Calculate the concentration of OH- and the p. H of the solution that is 0. 15 M in aqueous ammonia, NH 3, and 0. 30 M in ammonium nitrate, NH 4 NO 3. Kb = 1. 8 x 10 -5 R I C E 9
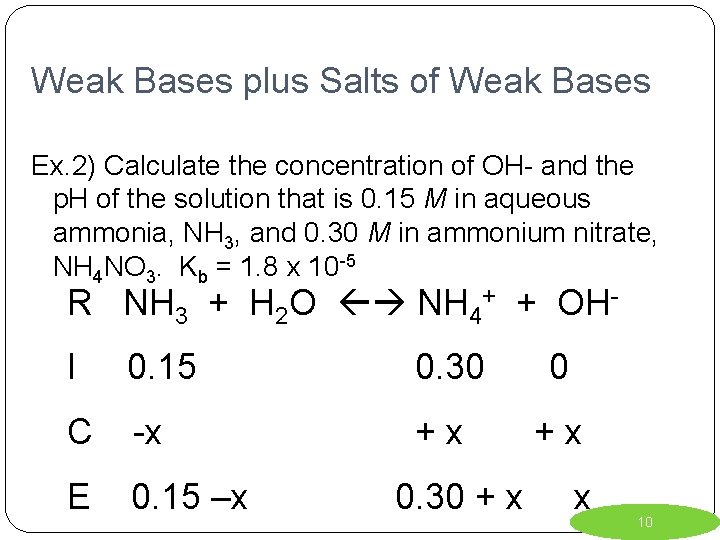
Weak Bases plus Salts of Weak Bases Ex. 2) Calculate the concentration of OH- and the p. H of the solution that is 0. 15 M in aqueous ammonia, NH 3, and 0. 30 M in ammonium nitrate, NH 4 NO 3. Kb = 1. 8 x 10 -5 R NH 3 + H 2 O NH 4+ + OHI 0. 15 0. 30 0 C -x +x +x E 0. 15 –x 0. 30 + x x 10
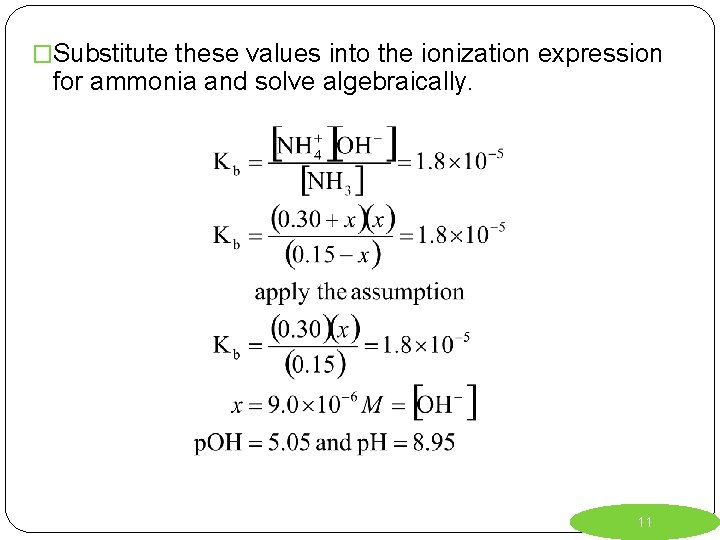
�Substitute these values into the ionization expression for ammonia and solve algebraically. 11
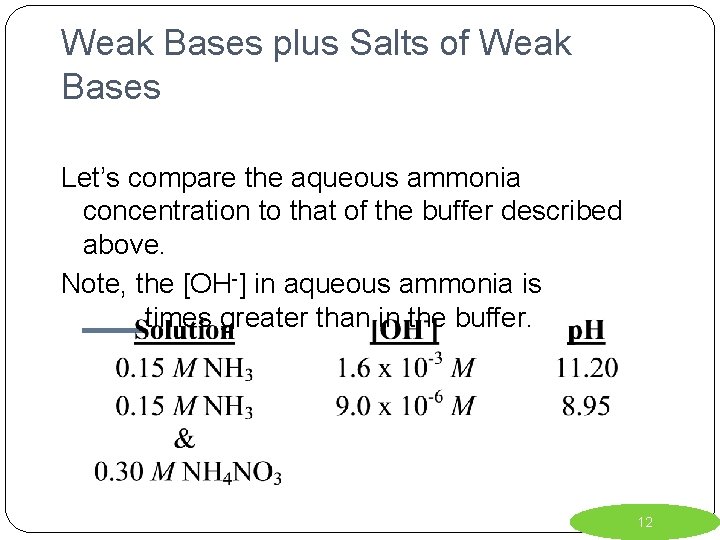
Weak Bases plus Salts of Weak Bases Let’s compare the aqueous ammonia concentration to that of the buffer described above. Note, the [OH-] in aqueous ammonia is ____times greater than in the buffer. 12
![Henderson-Hasselbach equation On your green sheets p. H = p. Ka + log [A-] Henderson-Hasselbach equation On your green sheets p. H = p. Ka + log [A-]](http://slidetodoc.com/presentation_image_h2/b50d25cb3bf586732229cb614711e1c3/image-13.jpg)
Henderson-Hasselbach equation On your green sheets p. H = p. Ka + log [A-] [HA] �Takes into account the dissociation factor of the weak acid (or weak base)
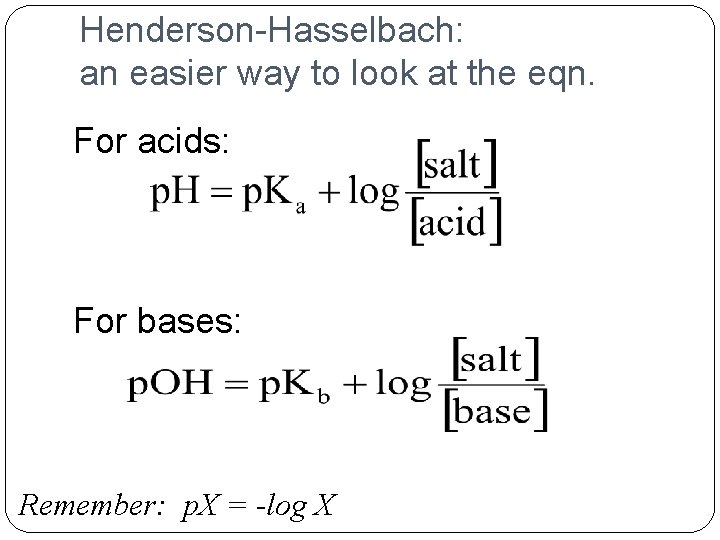
Henderson-Hasselbach: an easier way to look at the eqn. For acids: For bases: Remember: p. X = -log X
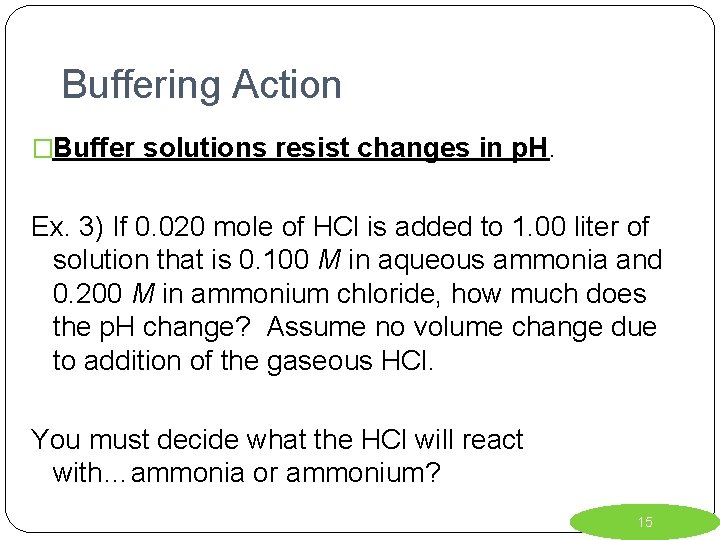
Buffering Action �Buffer solutions resist changes in p. H. Ex. 3) If 0. 020 mole of HCl is added to 1. 00 liter of solution that is 0. 100 M in aqueous ammonia and 0. 200 M in ammonium chloride, how much does the p. H change? Assume no volume change due to addition of the gaseous HCl. You must decide what the HCl will react with…ammonia or ammonium? 15

1 st ~ Calculate the p. H of the original buffer solution to find the initial p. H
2 nd ~ Calculate the concentration of all species after the addition of HCl. �HCl will react with some of the ammonia 17

3 rd ~ Now that you have the concentrations of our salt and base, you can calculate the new p. H. 18
4 th ~ Calculate the change in p. H. 19

Ex. 4) If 0. 020 mole of Na. OH is added to 1. 00 liter of solution that is 0. 100 M in aqueous ammonia and 0. 200 M in ammonium chloride, how much does the p. H change? Assume no volume change due to addition of the solid Na. OH. (Does the Na. OH react with the ammonia or the ammonium? ) 20
Preparation of Buffer Solutions Ex. 5) Calculate the concentration of H+ and the p. H of the solution prepared by mixing 200 m. L of 0. 150 M acetic acid and 100 m. L of 0. 100 M sodium hydroxide solutions. �Determine the molar amounts of acetic acid and sodium hydroxide (before reaction) �One of the reactants will be the limiting reagent, one will be in excess. 21
Preparation of Buffer Solutions �For _______ situations, it is sometimes important to prepare a buffer solution of a given p. H. Ex. 6) A) Find the number of moles of solid ammonium chloride, NH 4 Cl, that must be used to prepare 1. 00 L of a buffer solution that is 0. 10 M in aqueous ammonia, and that has a p. H of 9. 15 B) What mass is needed? 22
Acid-Base Indicators �_______ - point at which chemically equivalent amounts of acid and base have reacted �_______ - point at which chemical indicator changes color 23
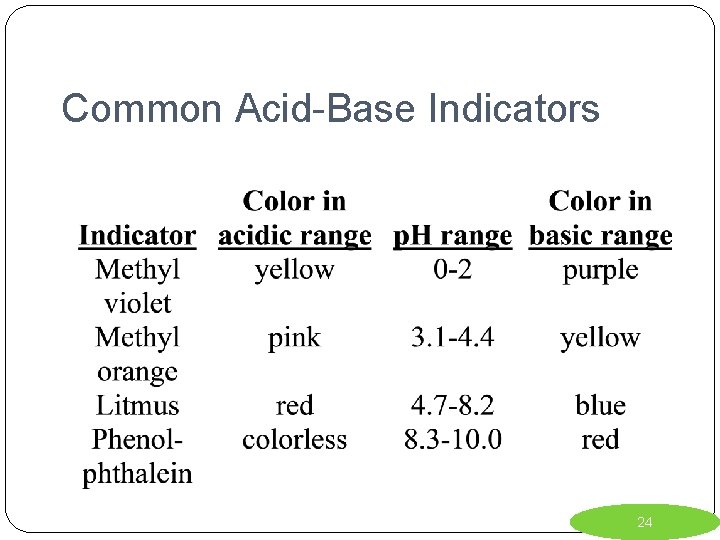
Common Acid-Base Indicators 24
Strong Acid/Strong Base Titration Curves �_______are graphs that show the p. H at various amounts of titrate added. Allows you to find the _______. �For Titration curves, Plot _______ of acid or base added in titration. 25
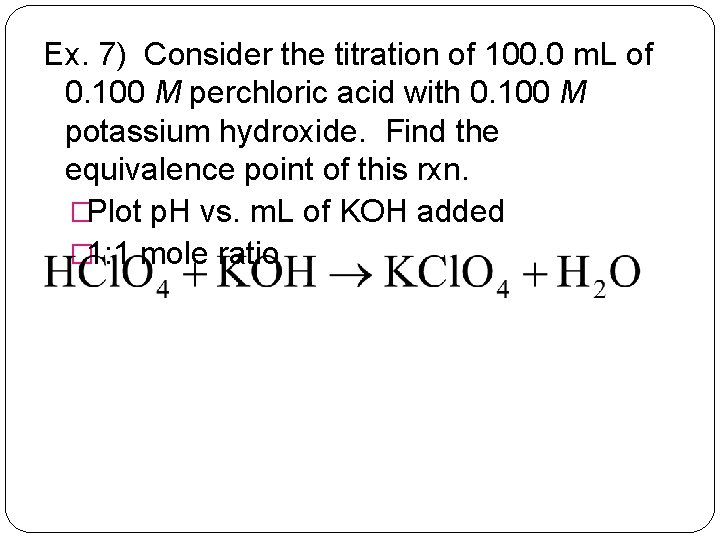
Ex. 7) Consider the titration of 100. 0 m. L of 0. 100 M perchloric acid with 0. 100 M potassium hydroxide. Find the equivalence point of this rxn. �Plot p. H vs. m. L of KOH added � 1: 1 mole ratio
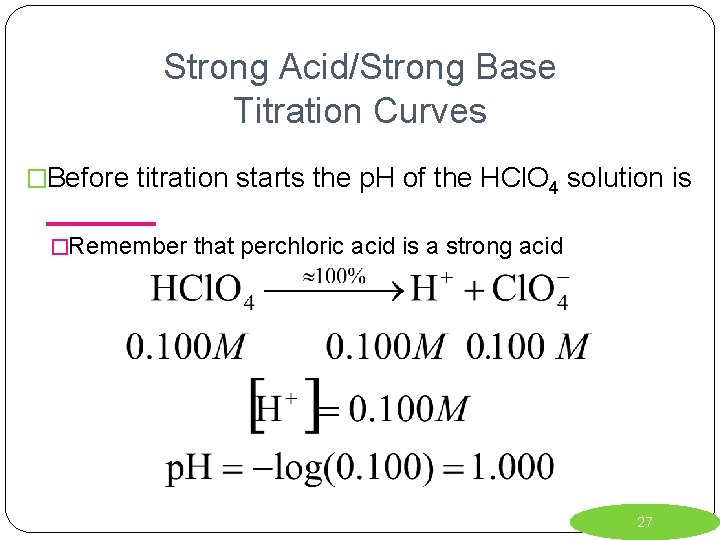
Strong Acid/Strong Base Titration Curves �Before titration starts the p. H of the HCl. O 4 solution is _______ �Remember that perchloric acid is a strong acid 27
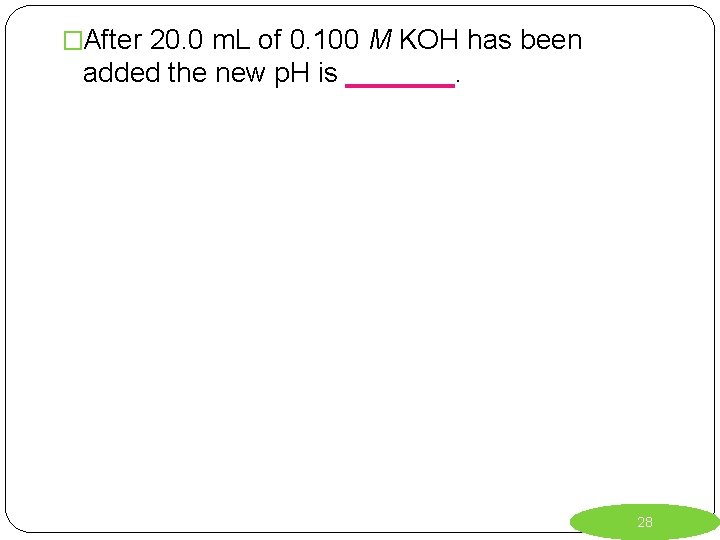
�After 20. 0 m. L of 0. 100 M KOH has been added the new p. H is _______. 28
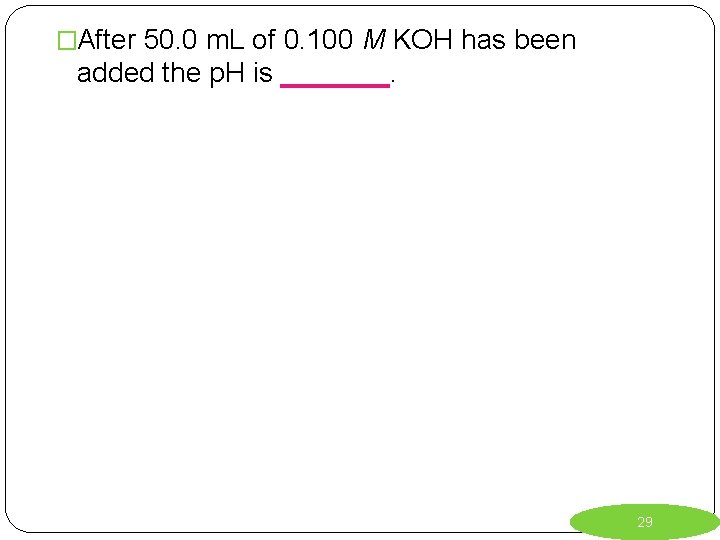
�After 50. 0 m. L of 0. 100 M KOH has been added the p. H is _______. 29
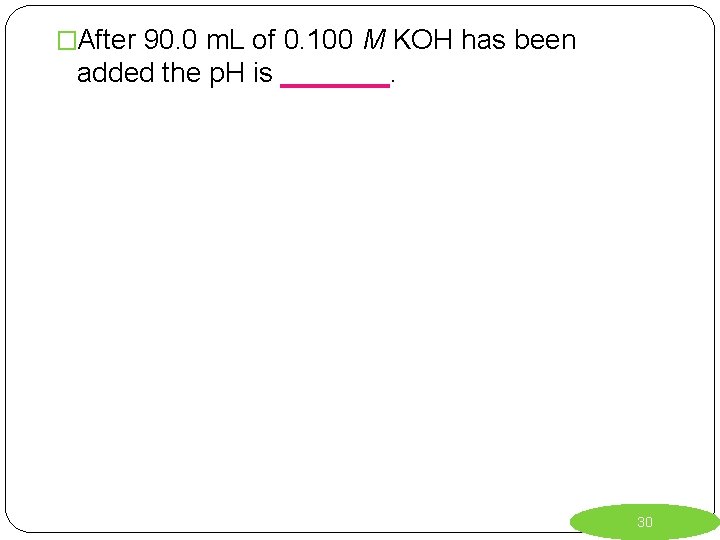
�After 90. 0 m. L of 0. 100 M KOH has been added the p. H is _______. 30
�After 100. 0 m. L of 0. 100 M KOH has been added the p. H is _______. 31
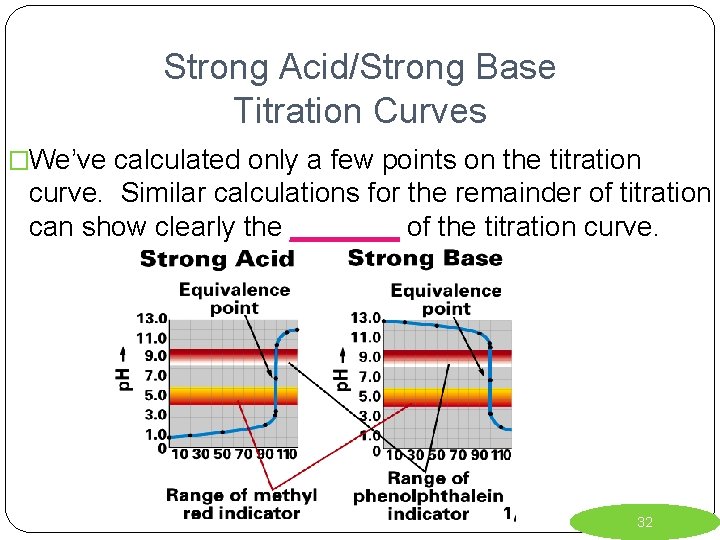
Strong Acid/Strong Base Titration Curves �We’ve calculated only a few points on the titration curve. Similar calculations for the remainder of titration can show clearly the _______ of the titration curve. 32
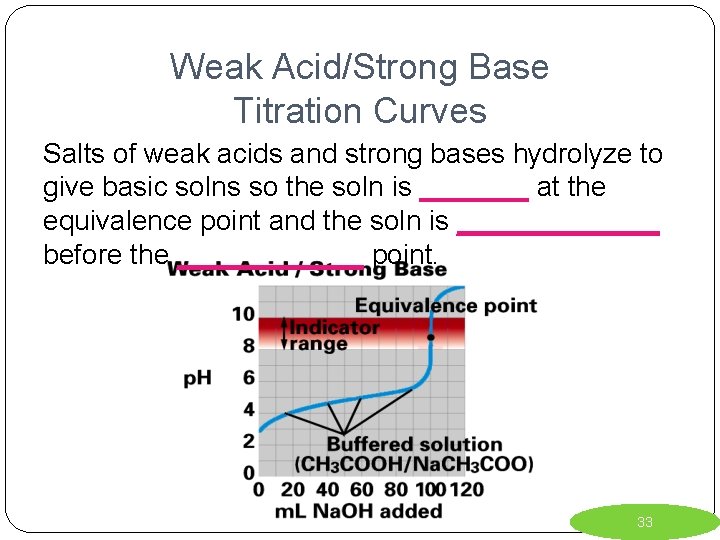
Weak Acid/Strong Base Titration Curves Salts of weak acids and strong bases hydrolyze to give basic solns so the soln is _______ at the equivalence point and the soln is _______ before the ______ point. 33
Strong Acid/Weak Base Titration Curves �Titration curves for Strong Acid/Weak Bases look similar to Strong Base/Weak Acid but they are inverted. The soln is _____ before the _______and is _______ at the ___________ 34
Weak Acid/Weak Base Titration Curves �Titration curves have _______vertical sections. �Solution is buffered both _______ and _______the equivalence point. �__________cannot be used. Instead you can measure the _______ in order to find the end point. �The math is complex, we will not worry about it in AP Chem. 35

More Fun Chemistry for you �Blood is slightly basic, having a p. H of 7. 35 to 7. 45. What chemical species causes our blood to be basic? How does our body regulate the p. H of blood? 36
- Slides: 36