Buffer systems RESPONSES TO ACIDOSIS AND ALKALOSIS Mechanisms

Buffer systems

RESPONSES TO: ACIDOSIS AND ALKALOSIS Mechanisms which protect the body against life-threatening changes in hydrogen ion concentration: 1) Buffering Systems in Body Fluids 2) Respiratory Responses 3) Renal Responses 4) Intracellular Shifts of Ions 2
1) Buffering systems in body fluids provide an immediate response to fluctuations in p. H 1) Phosphate 2) Protein 3) Bicarbonate Buffer System 3

Chemical buffers are able to react immediately (within milliseconds). Chemical buffers are the first line of defense for the body for fluctuations in p. H. 4
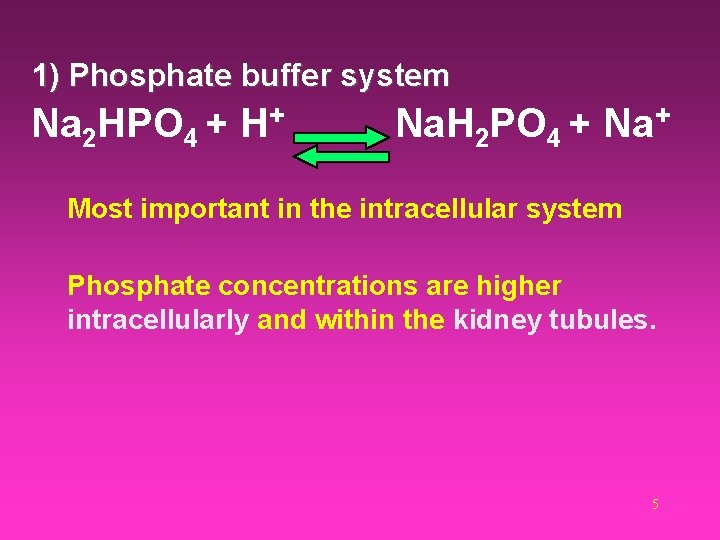
1) Phosphate buffer system Na 2 HPO 4 + H+ Na. H 2 PO 4 + Na+ Most important in the intracellular system Phosphate concentrations are higher intracellularly and within the kidney tubules. 5
2) Protein Buffer System Behaves as a buffer in both plasma and cells. Most important intracellular buffer (ICF). ICF The most plentiful buffer of the body. Hemoglobin is by far the most important protein buffer. 6
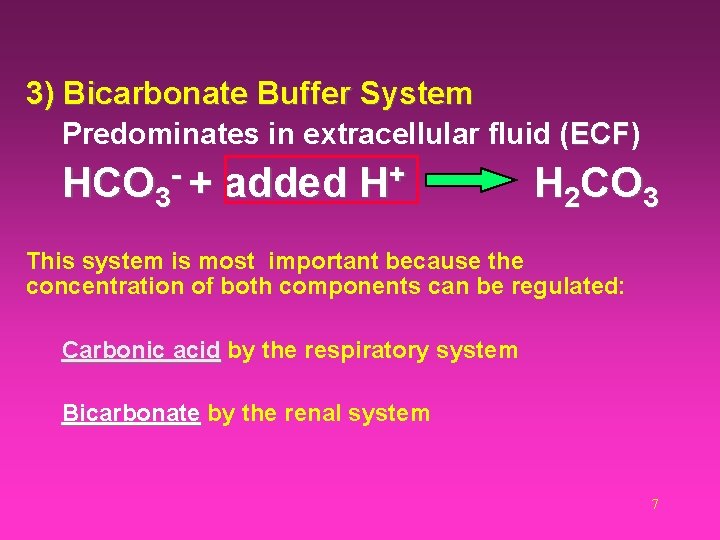
3) Bicarbonate Buffer System Predominates in extracellular fluid (ECF) ECF HCO 3 + added + H H 2 CO 3 This system is most important because the concentration of both components can be regulated: Carbonic acid by the respiratory system Bicarbonate by the renal system 7
2) Respiratory Responses Overall compensatory response is: Hyperventilation in response to increased CO 2 or H+ (low p. H). p. H Hypoventilation in response to decreased CO 2 or H+ (high p. H). p. H 8
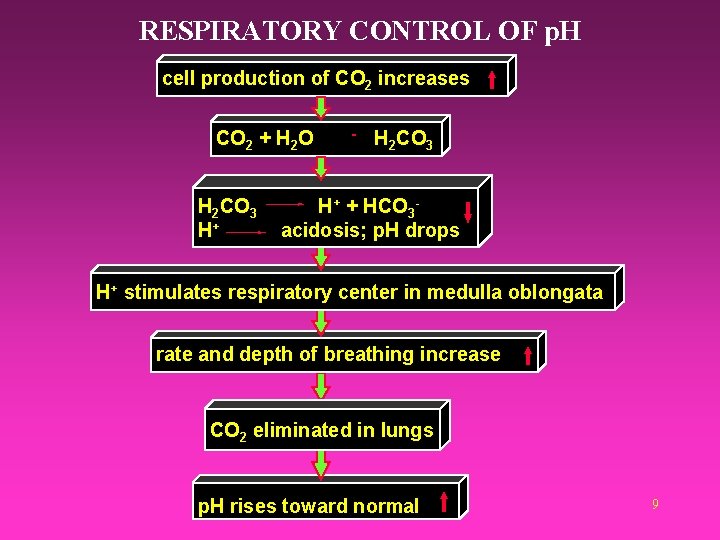
RESPIRATORY CONTROL OF p. H cell production of CO 2 increases CO 2 + H 2 O H 2 CO 3 H+ + HCO 3 acidosis; p. H drops H+ stimulates respiratory center in medulla oblongata rate and depth of breathing increase CO 2 eliminated in lungs p. H rises toward normal 9

3) RENAL RESPONSE The kidney compensates for Acid - Base imbalance within 24 hours and is responsible for long term control. The kidney in response: To Acidosis Retains bicarbonate ions and eliminates hydrogen ions. To Alkalosis Eliminates bicarbonate ions and retains hydrogen ions. 10
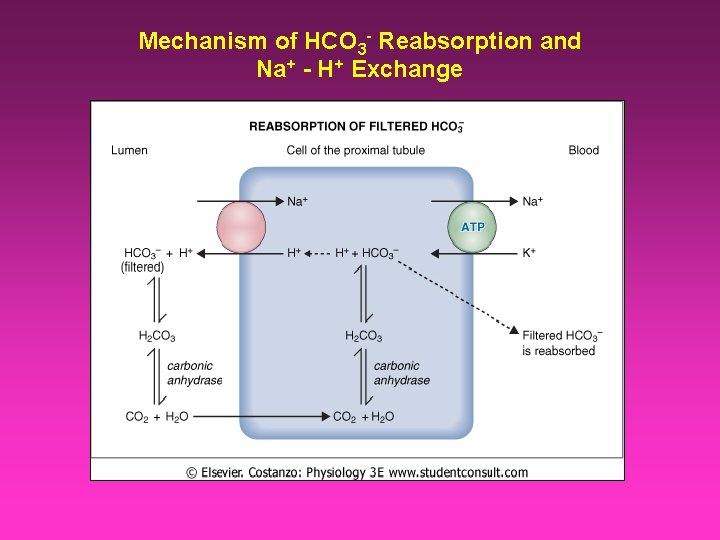
Mechanism of HCO 3 - Reabsorption and Na+ - H+ Exchange
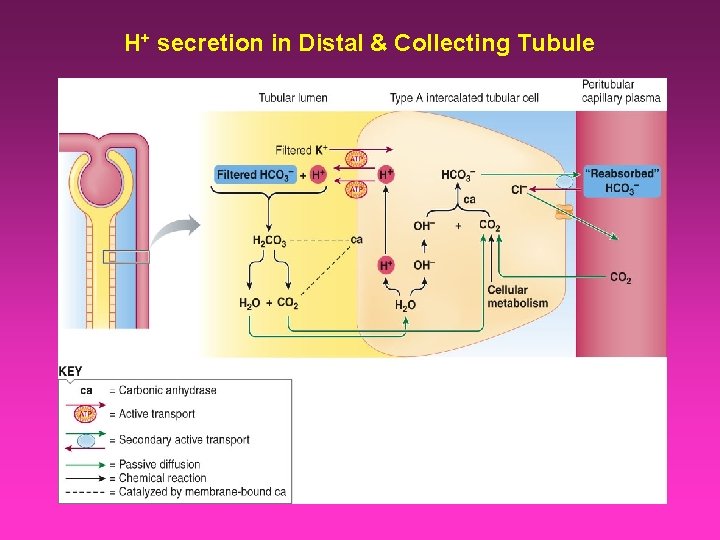
H+ secretion in Distal & Collecting Tubule
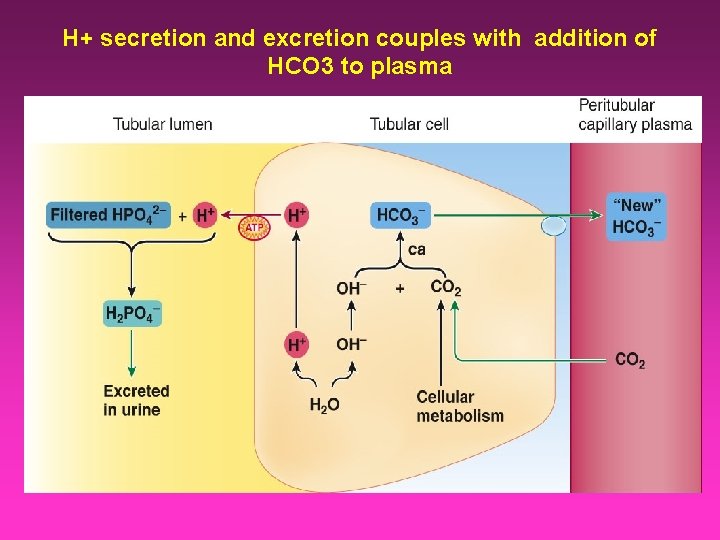
H+ secretion and excretion couples with addition of HCO 3 to plasma
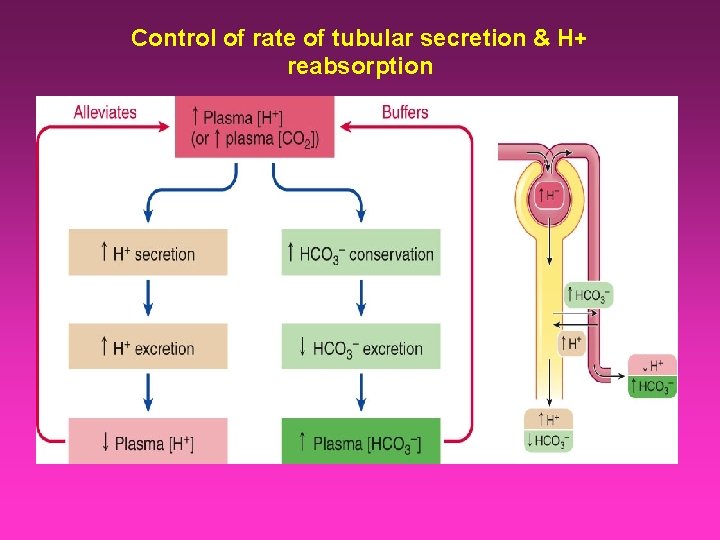
Control of rate of tubular secretion & H+ reabsorption
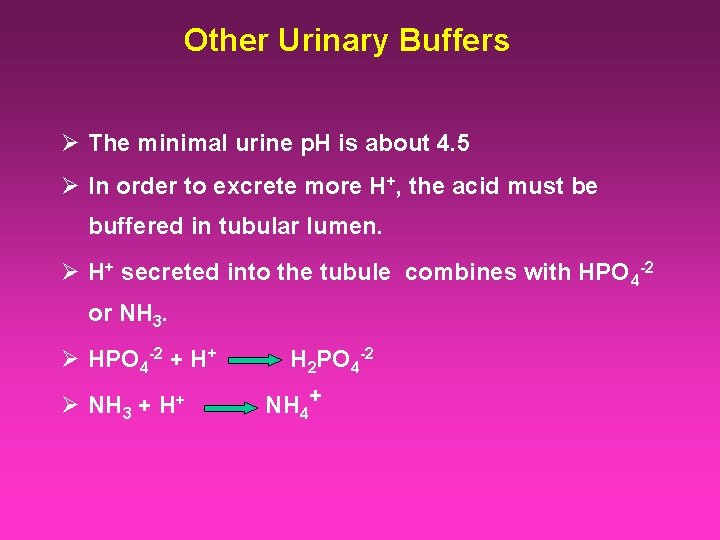
Other Urinary Buffers Ø The minimal urine p. H is about 4. 5 Ø In order to excrete more H+, the acid must be buffered in tubular lumen. Ø H+ secreted into the tubule combines with HPO 4 -2 or NH 3. Ø HPO 4 -2 + H+ Ø NH 3 + H+ H 2 PO 4 -2 NH 4+
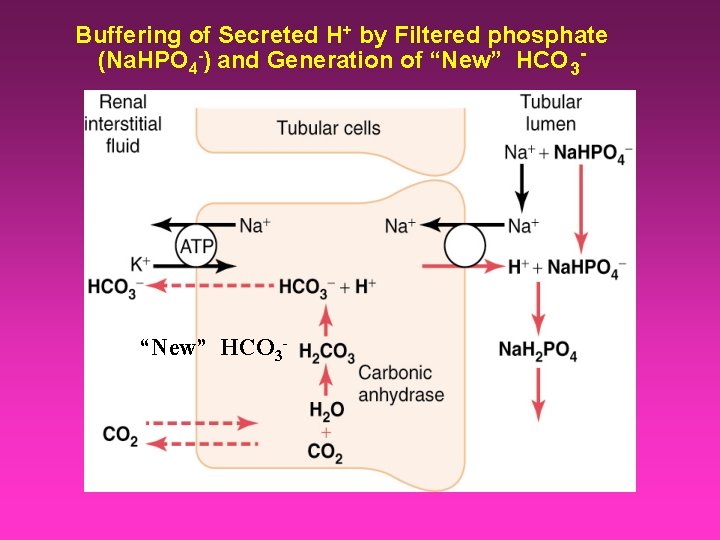
Buffering of Secreted H+ by Filtered phosphate (Na. HPO 4 -) and Generation of “New” HCO 3 -

Glutamine is the most abundant free amino acid that: 1 - Help in protein synthesis. 2 - regulate acid –base balance in the kidney by producing ammonium. During metabolic acidosis, the kidney becomes the major site for glutamine extraction and catabolism.
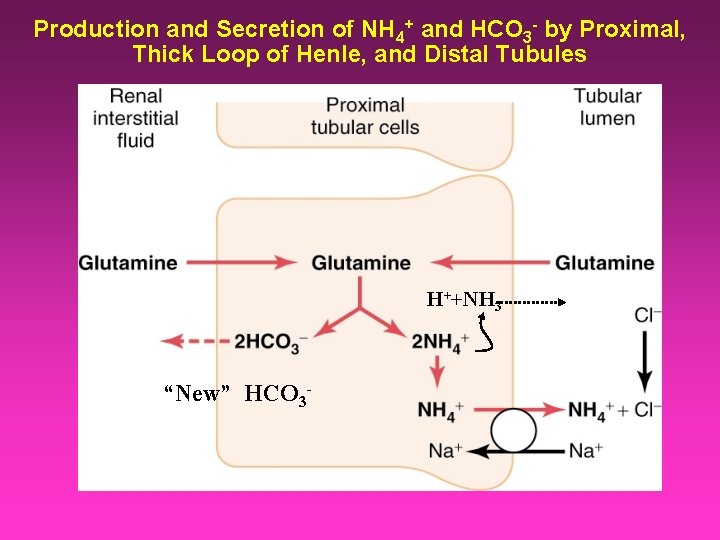
Production and Secretion of NH 4+ and HCO 3 - by Proximal, Thick Loop of Henle, and Distal Tubules H++NH 3 “New” HCO 3 -

4) Intracellular Shifts of Ions Hyperkalemia Is generally associated with acidosis. Accompanied by a shift of H+ ions into cells and K+ ions out of the cell to maintain electrical neutrality. Hypokalemia Is generally associated with reciprocal exchanges of H+ and K+ in the opposite direction. Associated with alkalosis. 19
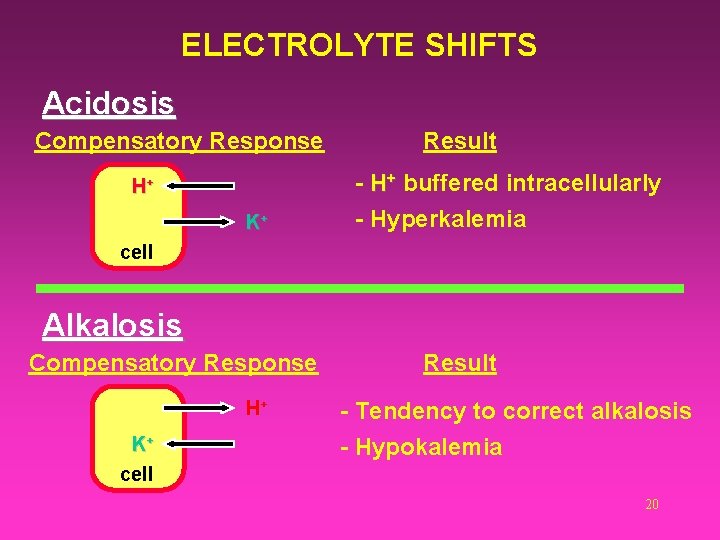
ELECTROLYTE SHIFTS Acidosis Compensatory Response H+ K+ Result - H+ buffered intracellularly - Hyperkalemia cell Alkalosis Compensatory Response H+ K+ cell Result - Tendency to correct alkalosis - Hypokalemia 20
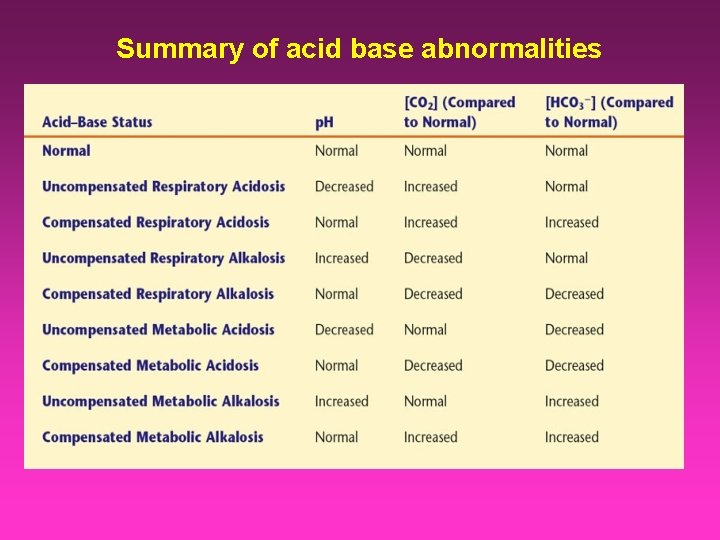
Summary of acid base abnormalities
- Slides: 21