Bubble Point and dew point Calculations The basic
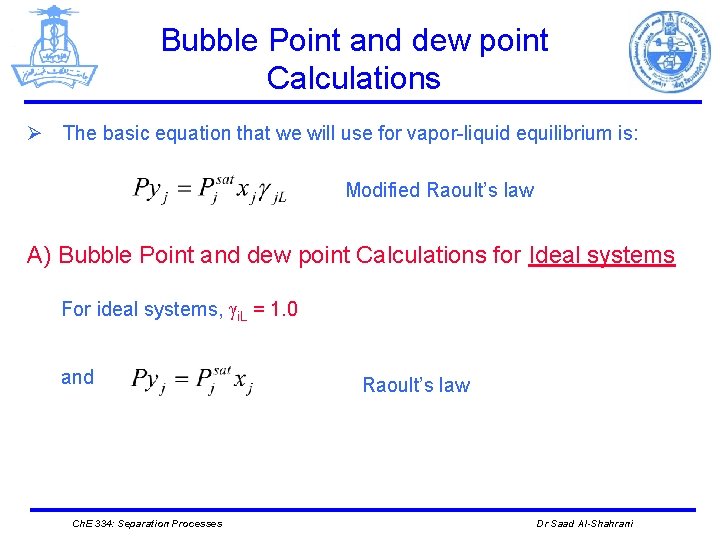
Bubble Point and dew point Calculations Ø The basic equation that we will use for vapor-liquid equilibrium is: Modified Raoult’s law A) Bubble Point and dew point Calculations for Ideal systems For ideal systems, i. L = 1. 0 and Ch. E 334: Separation Processes Raoult’s law Dr Saad Al-Shahrani
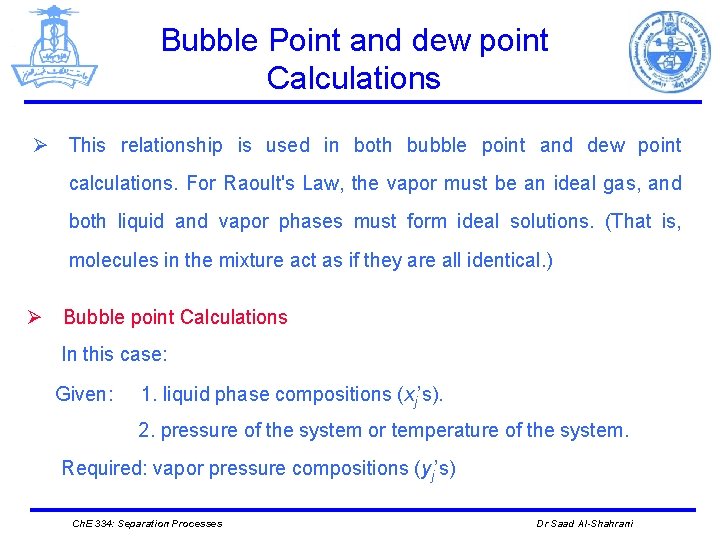
Bubble Point and dew point Calculations Ø This relationship is used in both bubble point and dew point calculations. For Raoult's Law, the vapor must be an ideal gas, and both liquid and vapor phases must form ideal solutions. (That is, molecules in the mixture act as if they are all identical. ) Ø Bubble point Calculations In this case: Given: 1. liquid phase compositions (xj’s). 2. pressure of the system or temperature of the system. Required: vapor pressure compositions (yj’s) Ch. E 334: Separation Processes Dr Saad Al-Shahrani
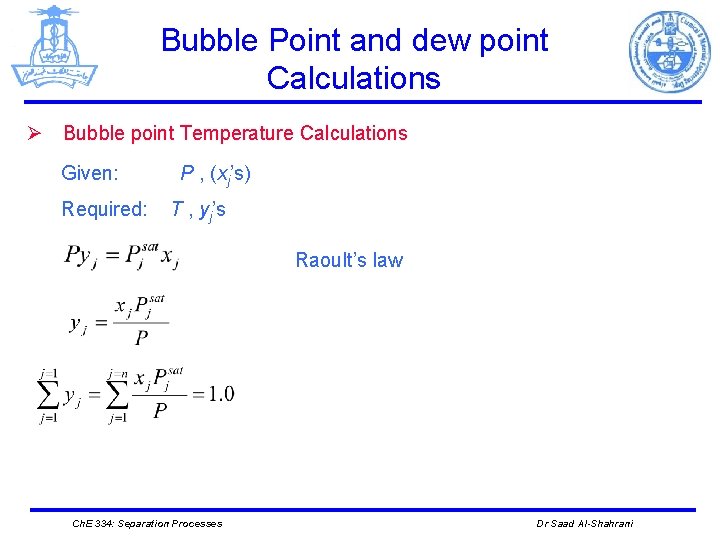
Bubble Point and dew point Calculations Ø Bubble point Temperature Calculations Given: Required: P , (xj’s) T , yj’s Raoult’s law Ch. E 334: Separation Processes Dr Saad Al-Shahrani
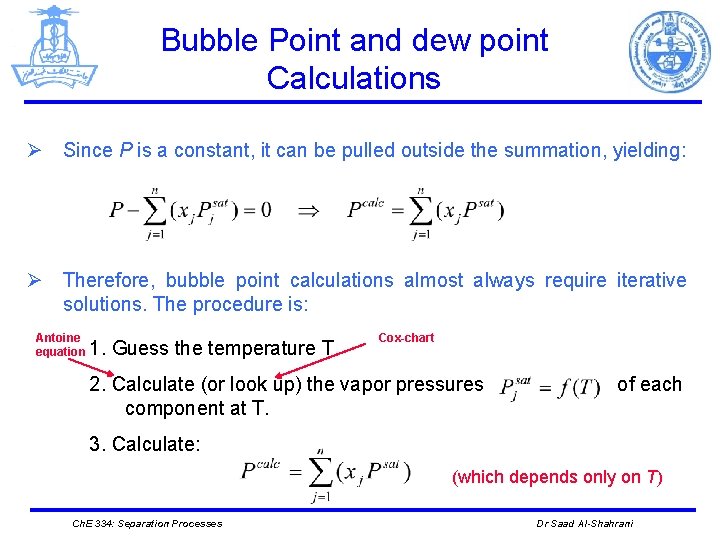
Bubble Point and dew point Calculations Ø Since P is a constant, it can be pulled outside the summation, yielding: Ø Therefore, bubble point calculations almost always require iterative solutions. The procedure is: Antoine equation 1. Guess the temperature T. Cox-chart 2. Calculate (or look up) the vapor pressures component at T. of each 3. Calculate: (which depends only on T) Ch. E 334: Separation Processes Dr Saad Al-Shahrani
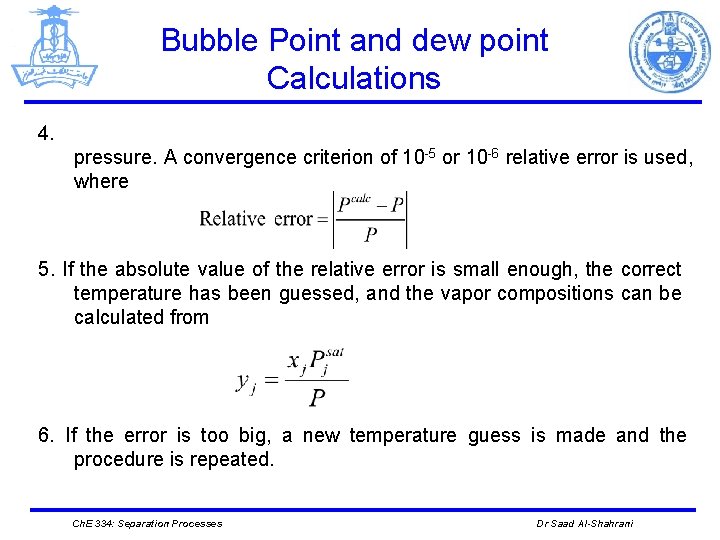
Bubble Point and dew point Calculations 4. pressure. A convergence criterion of 10 -5 or 10 -6 relative error is used, where 5. If the absolute value of the relative error is small enough, the correct temperature has been guessed, and the vapor compositions can be calculated from 6. If the error is too big, a new temperature guess is made and the procedure is repeated. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Bubble Point and dew point Calculations Example: The liquid in a process vessel contains 40 mole percent benzene, 35 mole percent toluene, and 25 mole percent o-xylene. The total pressure in the vessel is 2 atmospheres. What is the temperature in the vessel, and what is the composition of the vapor? solution P=2 atm Vapor phase Let x 1 be the mole fraction of benzene in the liquid, x 2 be the mole fraction toluene, and x 3 be the mole fraction o-xylene. P= 1, 520 mm. Hg (2 atm) Liquid phase T=? Guess: T=120 o. C. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
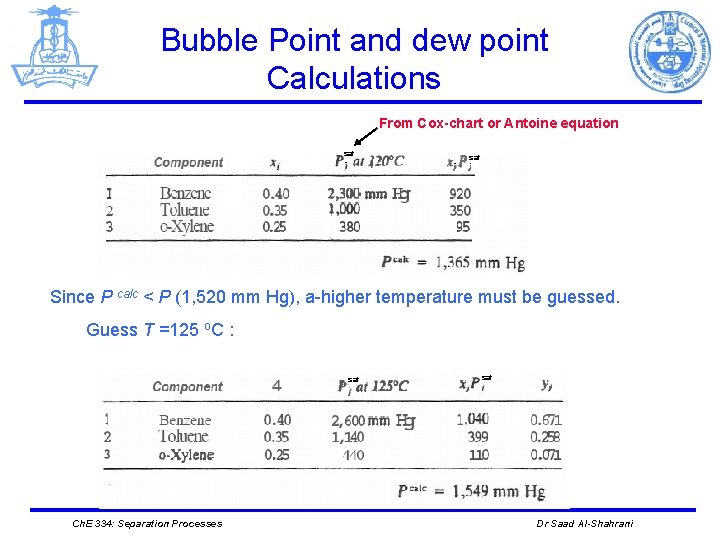
Bubble Point and dew point Calculations From Cox-chart or Antoine equation sat Since P calc < P (1, 520 mm Hg), a-higher temperature must be guessed. Guess T =125 o. C : sat Ch. E 334: Separation Processes sat Dr Saad Al-Shahrani
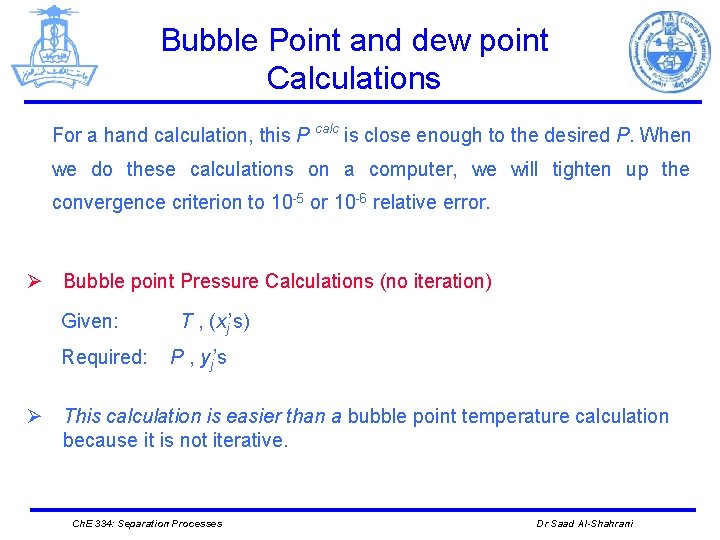
Bubble Point and dew point Calculations For a hand calculation, this P calc is close enough to the desired P. When we do these calculations on a computer, we will tighten up the convergence criterion to 10 -5 or 10 -6 relative error. Ø Bubble point Pressure Calculations (no iteration) Given: Required: T , (xj’s) P , yj’s Ø This calculation is easier than a bubble point temperature calculation because it is not iterative. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
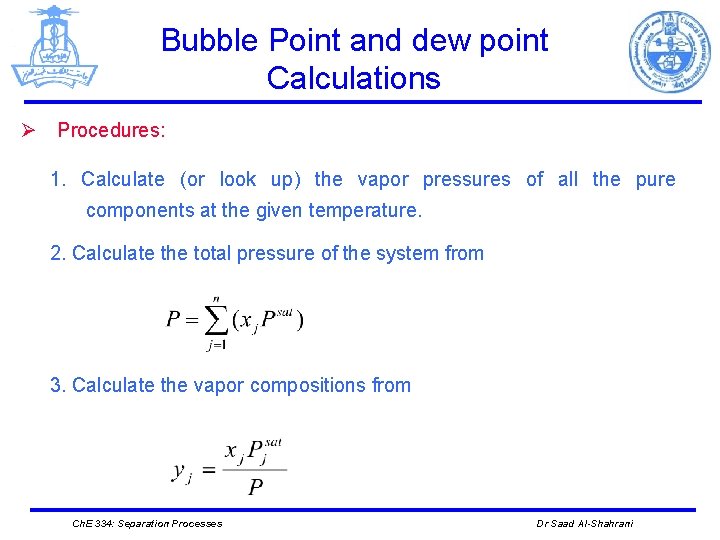
Bubble Point and dew point Calculations Ø Procedures: 1. Calculate (or look up) the vapor pressures of all the pure components at the given temperature. 2. Calculate the total pressure of the system from 3. Calculate the vapor compositions from Ch. E 334: Separation Processes Dr Saad Al-Shahrani
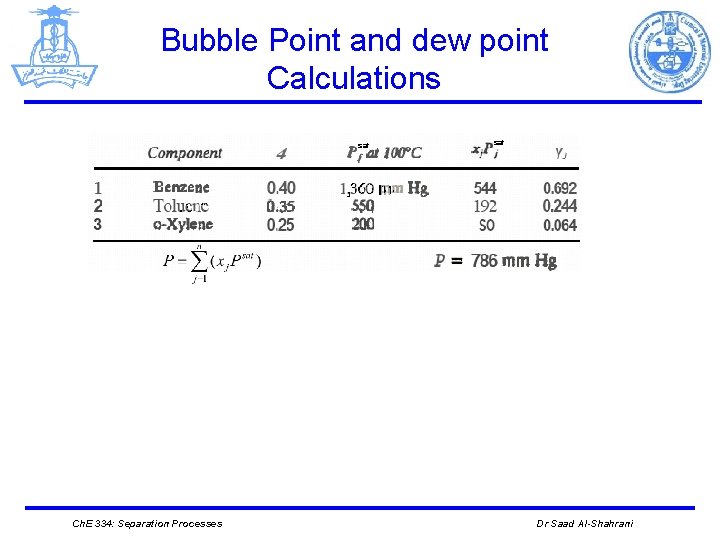
Bubble Point and dew point Calculations Example A liquid mixture of 40 mole percent benzene, 35 mole percent toluene, and 25 mole percent o-xylene is held at 100 °C in a closed vessel. What is the pressure in the vessel, and what is the composition of the vapor that is in equilibrium with the liquid? solution The vapor pressures of the pure components are looked up at 100 o. C. At this temperature, they are 1, 360 Hg for benzene, 550 mm Hg for toluene, and 200 mm Hg for o-xylene. Thus. we have: Ch. E 334: Separation Processes Dr Saad Al-Shahrani
Bubble Point and dew point Calculations sat Ch. E 334: Separation Processes sat Dr Saad Al-Shahrani
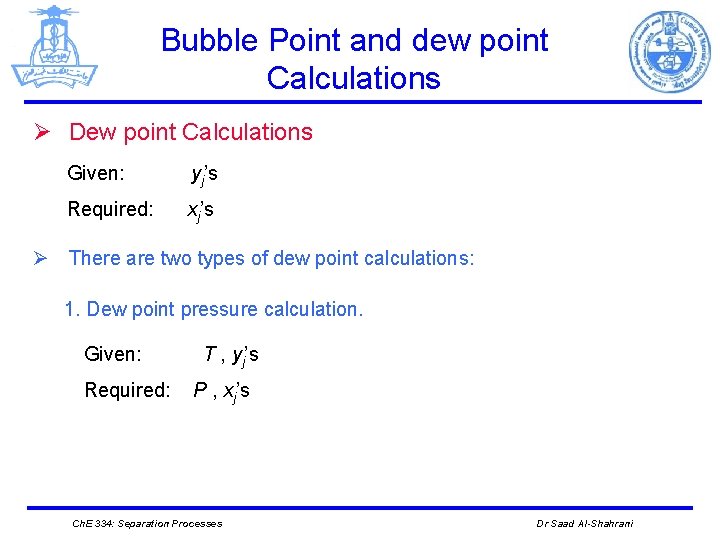
Bubble Point and dew point Calculations Ø Dew point Calculations Given: yj’s Required: xj’s Ø There are two types of dew point calculations: 1. Dew point pressure calculation. Given: Required: T , yj’s P , xj’s Ch. E 334: Separation Processes Dr Saad Al-Shahrani
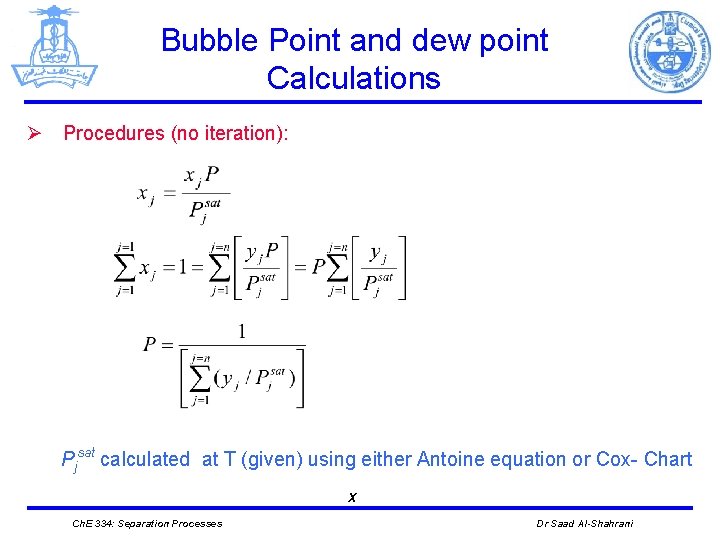
Bubble Point and dew point Calculations Ø Procedures (no iteration): Pjsat calculated at T (given) using either Antoine equation or Cox- Chart X Ch. E 334: Separation Processes Dr Saad Al-Shahrani
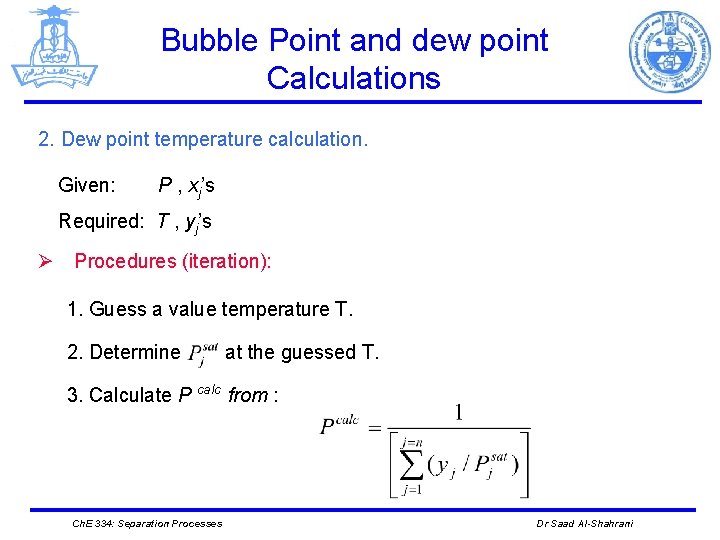
Bubble Point and dew point Calculations 2. Dew point temperature calculation. Given: P , xj’s Required: T , yj’s Ø Procedures (iteration): 1. Guess a value temperature T. 2. Determine at the guessed T. 3. Calculate P calc from : Ch. E 334: Separation Processes Dr Saad Al-Shahrani
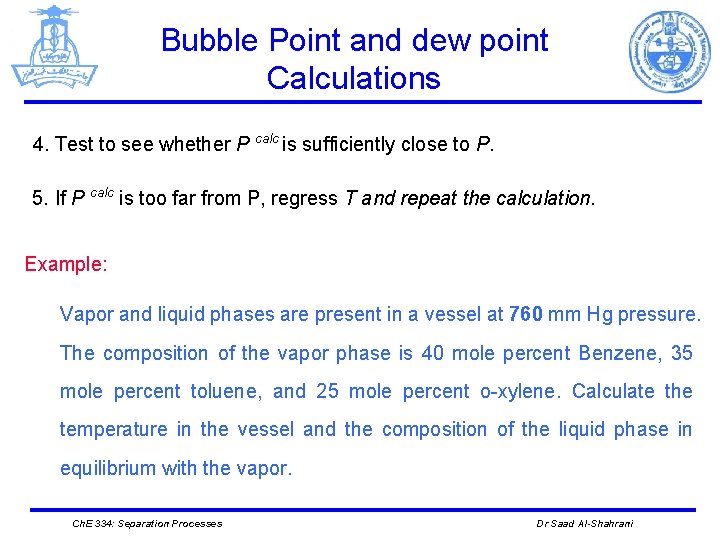
Bubble Point and dew point Calculations 4. Test to see whether P calc is sufficiently close to P. 5. If P calc is too far from P, regress T and repeat the calculation. Example: Vapor and liquid phases are present in a vessel at 760 mm Hg pressure. The composition of the vapor phase is 40 mole percent Benzene, 35 mole percent toluene, and 25 mole percent o-xylene. Calculate the temperature in the vessel and the composition of the liquid phase in equilibrium with the vapor. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
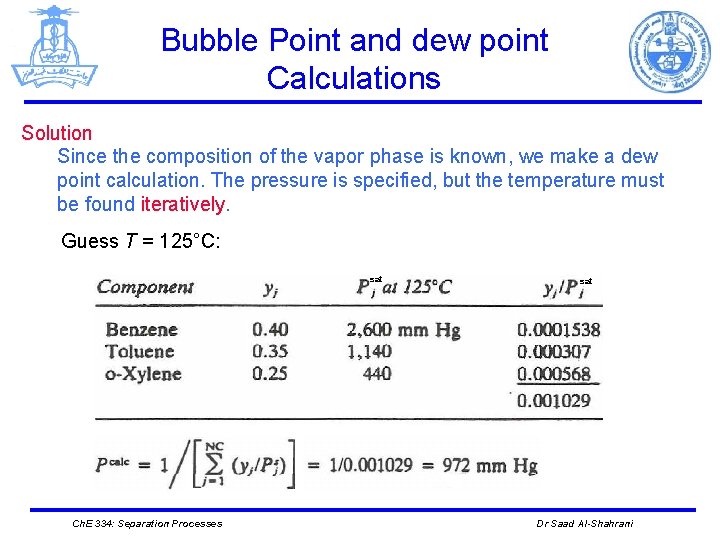
Bubble Point and dew point Calculations Solution Since the composition of the vapor phase is known, we make a dew point calculation. The pressure is specified, but the temperature must be found iteratively. Guess T = 125°C: sat Ch. E 334: Separation Processes sat Dr Saad Al-Shahrani
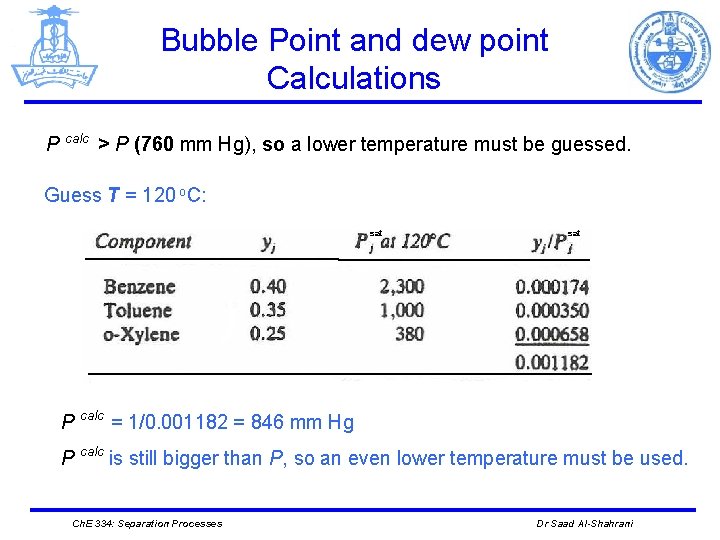
Bubble Point and dew point Calculations P calc > P (760 mm Hg), so a lower temperature must be guessed. Guess T = 120 o. C: sat P calc = 1/0. 001182 = 846 mm Hg P calc is still bigger than P, so an even lower temperature must be used. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
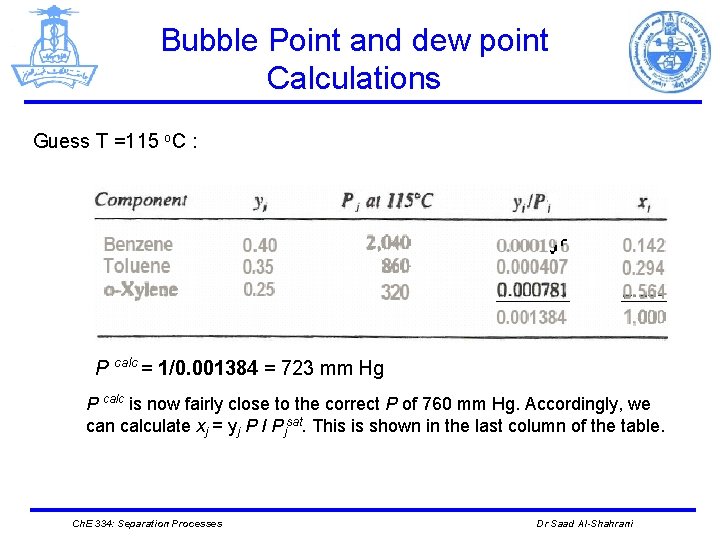
Bubble Point and dew point Calculations Guess T =115 o. C : P calc = 1/0. 001384 = 723 mm Hg P calc is now fairly close to the correct P of 760 mm Hg. Accordingly, we can calculate xj = yj P I Pjsat. This is shown in the last column of the table. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
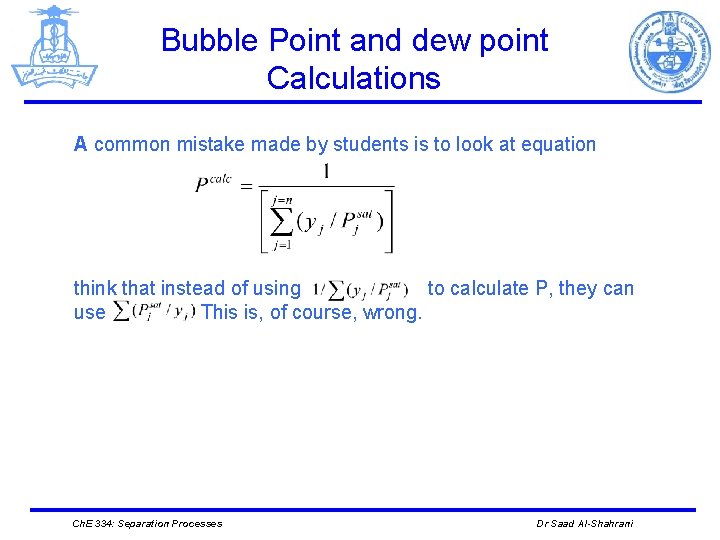
Bubble Point and dew point Calculations A common mistake made by students is to look at equation think that instead of using to calculate P, they can use This is, of course, wrong. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
- Slides: 19