Bring your laboratory notebook safety glasses and wear

Bring your laboratory notebook, safety glasses and wear closed toe shoes to lab tomorrow!!! Read Laboratory Exercise: Macromolecules beginning on page 57 of your lab manual

24 September 2012 26 September 2012
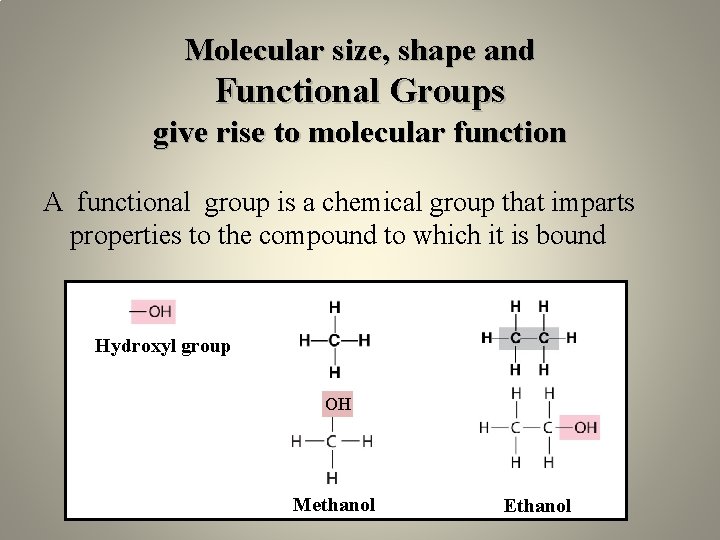
Molecular size, shape and Functional Groups give rise to molecular function A functional group is a chemical group that imparts properties to the compound to which it is bound Hydroxyl group Methane OH Ethane Methanol Ethanol
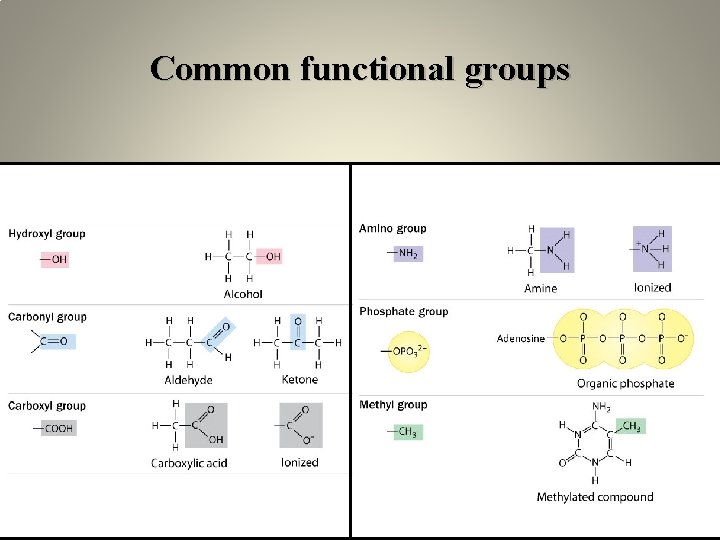
Common functional groups
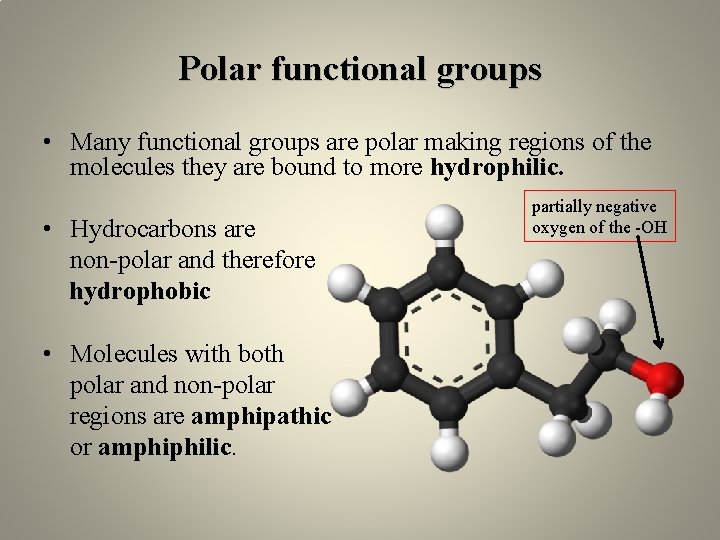
Polar functional groups • Many functional groups are polar making regions of the molecules they are bound to more hydrophilic. • Hydrocarbons are non-polar and therefore hydrophobic • Molecules with both polar and non-polar regions are amphipathic or amphiphilic. partially negative oxygen of the -OH
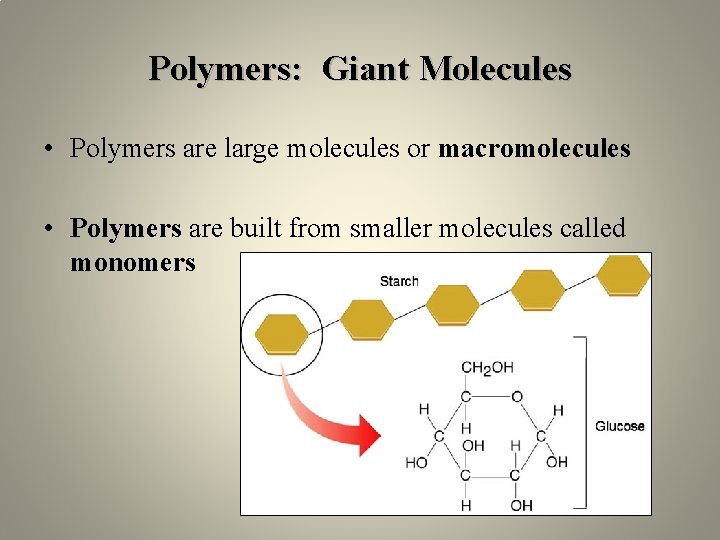
Polymers: Giant Molecules • Polymers are large molecules or macromolecules • Polymers are built from smaller molecules called monomers
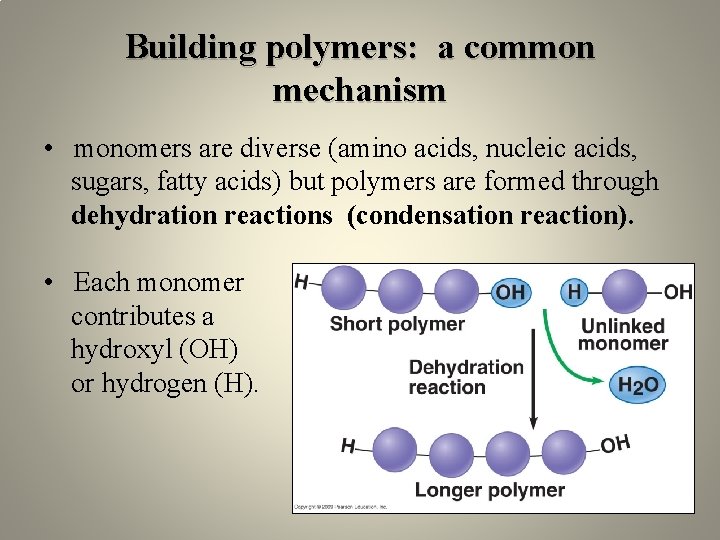
Building polymers: a common mechanism • monomers are diverse (amino acids, nucleic acids, sugars, fatty acids) but polymers are formed through dehydration reactions (condensation reaction). • Each monomer contributes a hydroxyl (OH) or hydrogen (H).
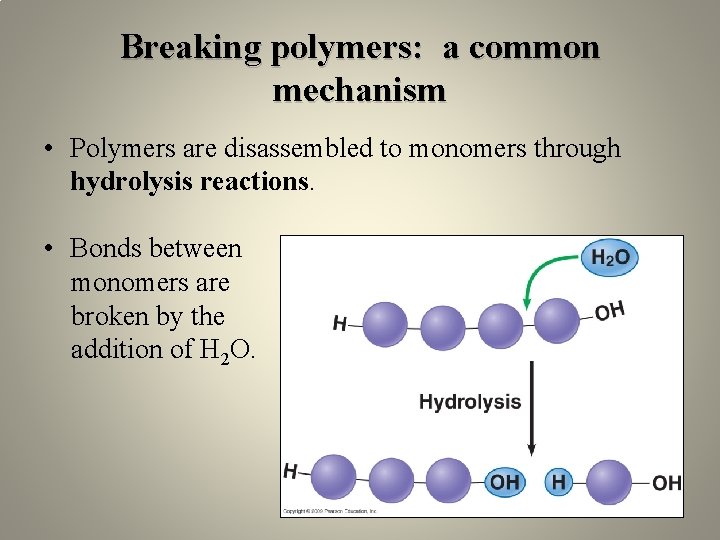
Breaking polymers: a common mechanism • Polymers are disassembled to monomers through hydrolysis reactions. • Bonds between monomers are broken by the addition of H 2 O.
Four main classes of molecules for life Proteins Lipids Carbohydrates Nucleic Acids

Carbohydrates • single sugars in combinations or alone • carbon, oxygen and hydrogen
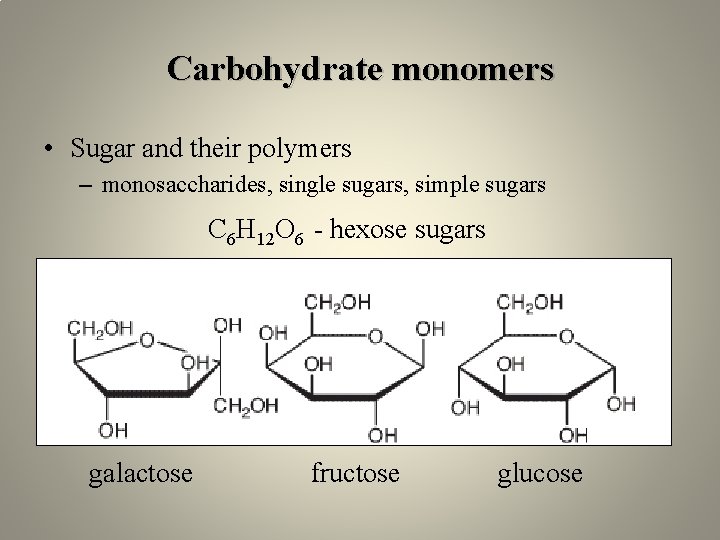
Carbohydrate monomers • Sugar and their polymers – monosaccharides, single sugars, simple sugars C 6 H 12 O 6 - hexose sugars galactose fructose glucose
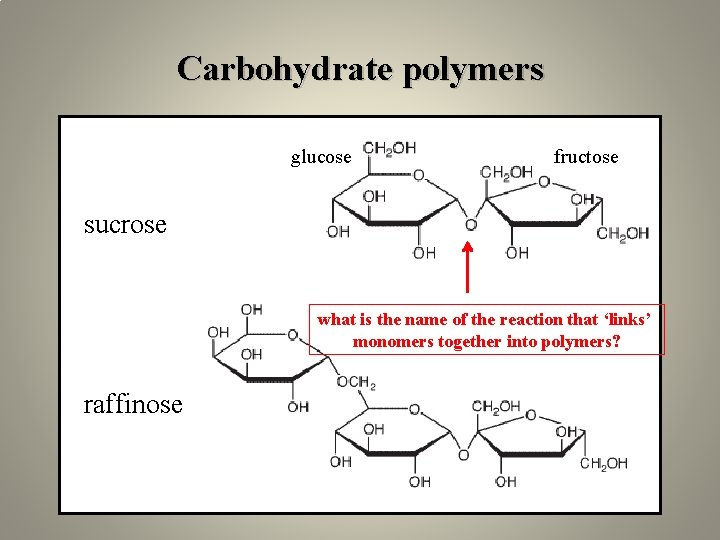
Carbohydrate polymers glucose fructose sucrose what is the name of the reaction that ‘links’ monomers together into polymers? raffinose
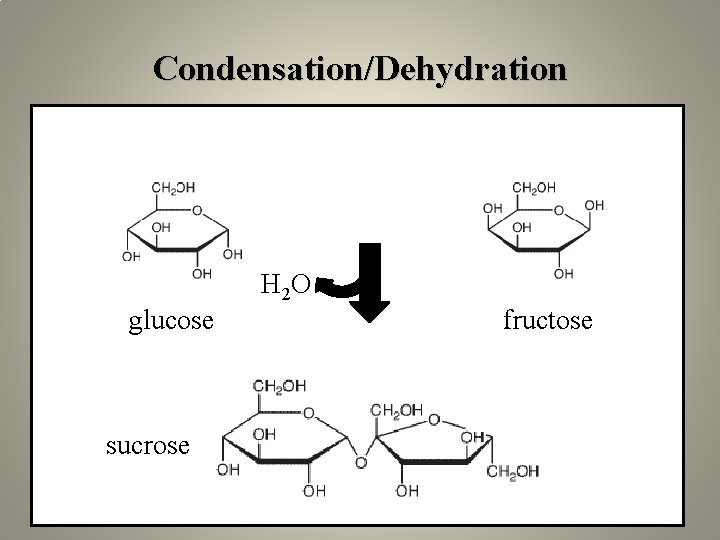
Condensation/Dehydration H 2 O glucose sucrose fructose
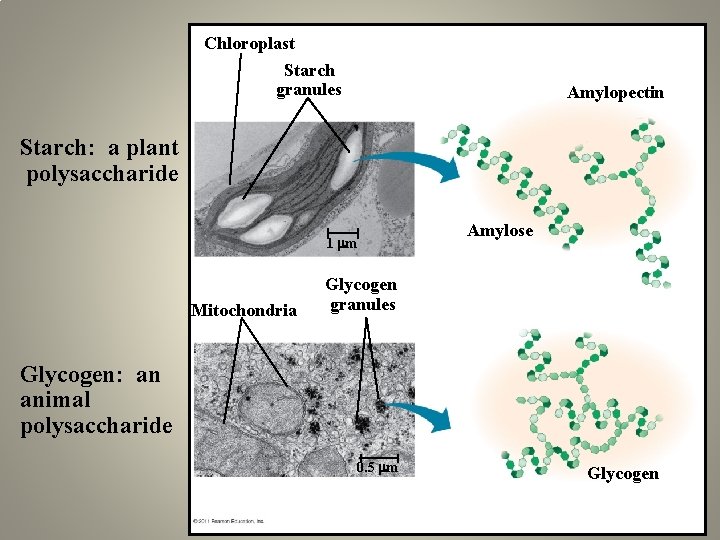
Chloroplast Starch granules Amylopectin Starch: a plant polysaccharide Amylose 1 m Mitochondria Glycogen granules Glycogen: an animal polysaccharide 0. 5 m Glycogen
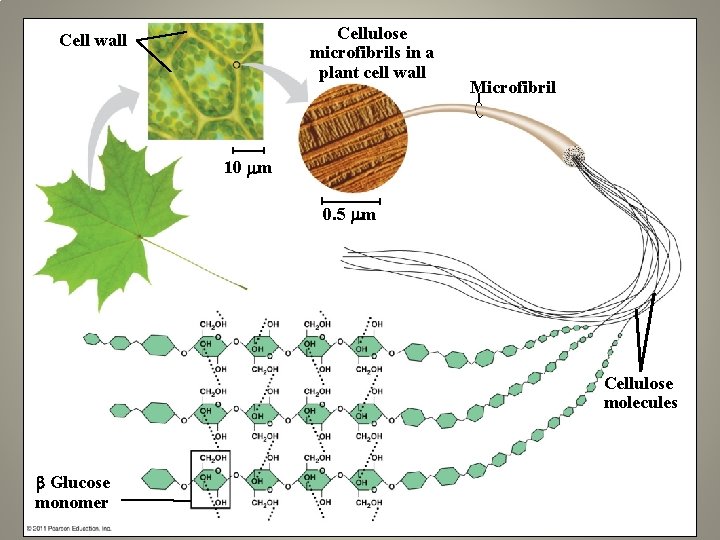
Cellulose microfibrils in a plant cell wall Cell wall Microfibril 10 m 0. 5 m Cellulose molecules Glucose monomer
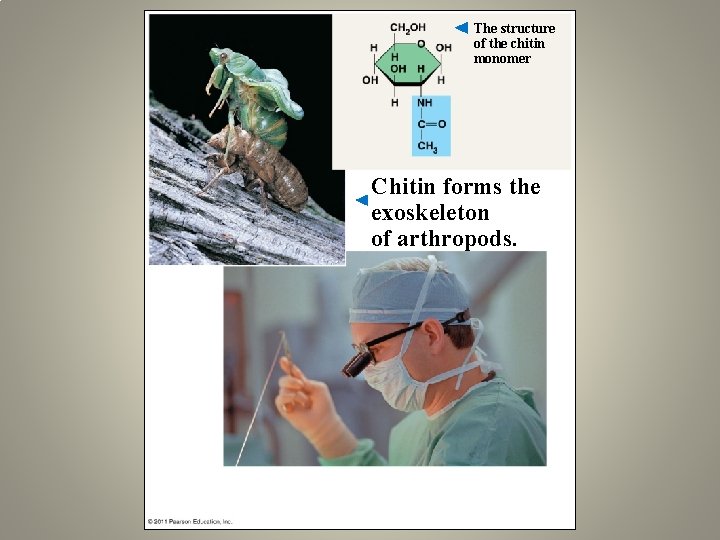
The structure of the chitin monomer Chitin forms the exoskeleton of arthropods.
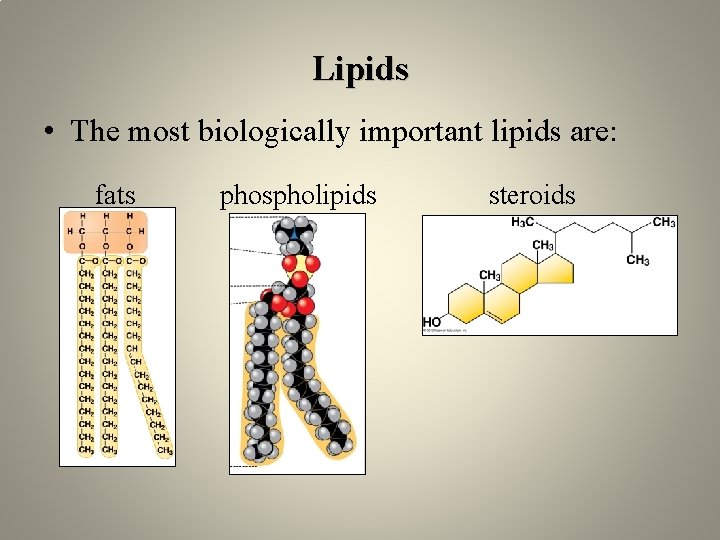
Lipids • The most biologically important lipids are: fats phospholipids steroids
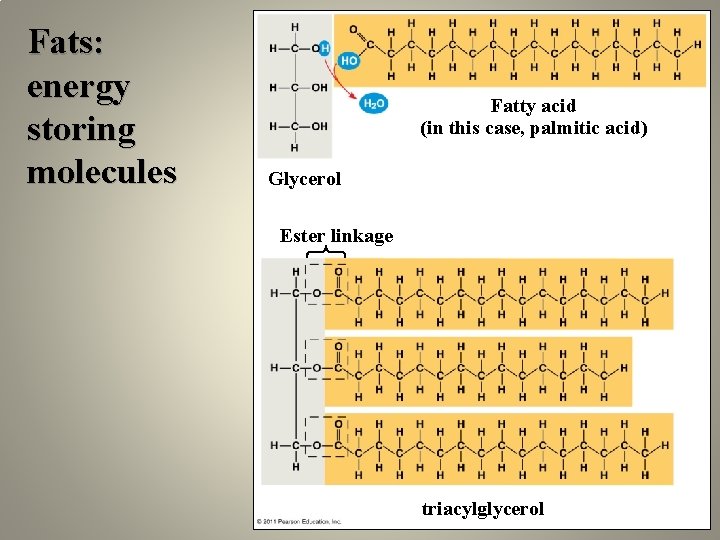
Fats: energy storing molecules Fatty acid (in this case, palmitic acid) Glycerol Ester linkage triacylglycerol
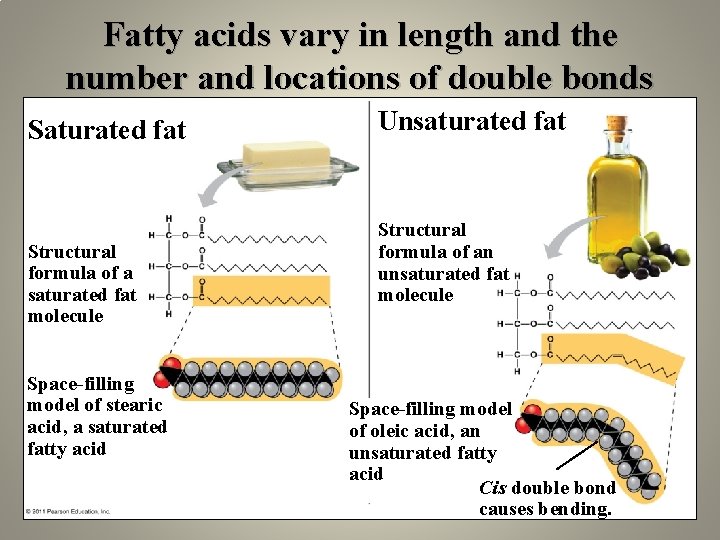
Fatty acids vary in length and the number and locations of double bonds Saturated fat Structural formula of a saturated fat molecule Space-filling model of stearic acid, a saturated fatty acid Unsaturated fat Structural formula of an unsaturated fat molecule Space-filling model of oleic acid, an unsaturated fatty acid Cis double bond causes bending.
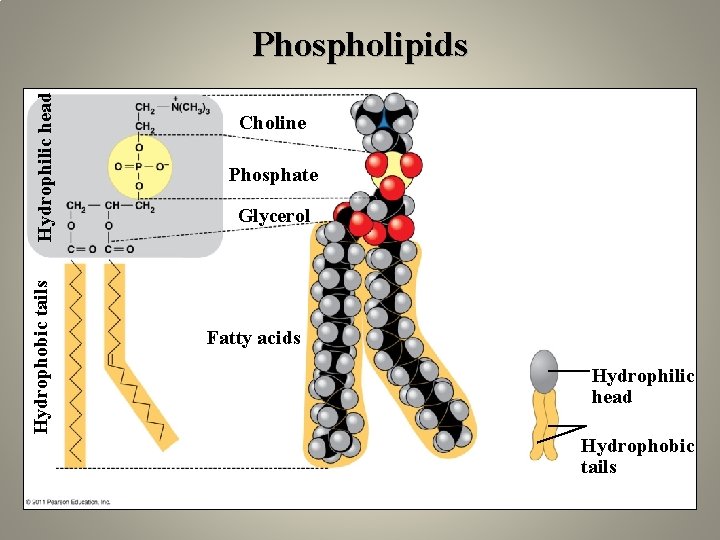
Hydrophobic tails Hydrophilic head Phospholipids Choline Phosphate Glycerol Fatty acids Hydrophilic head Hydrophobic tails
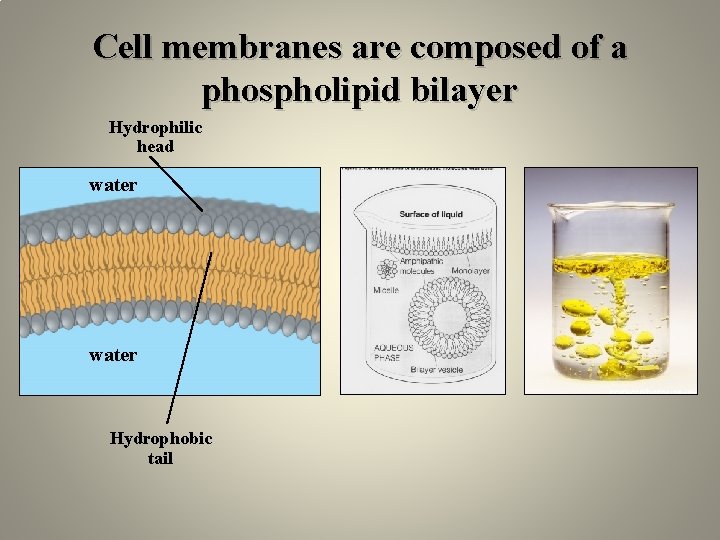
Cell membranes are composed of a phospholipid bilayer Hydrophilic head water Hydrophobic tail
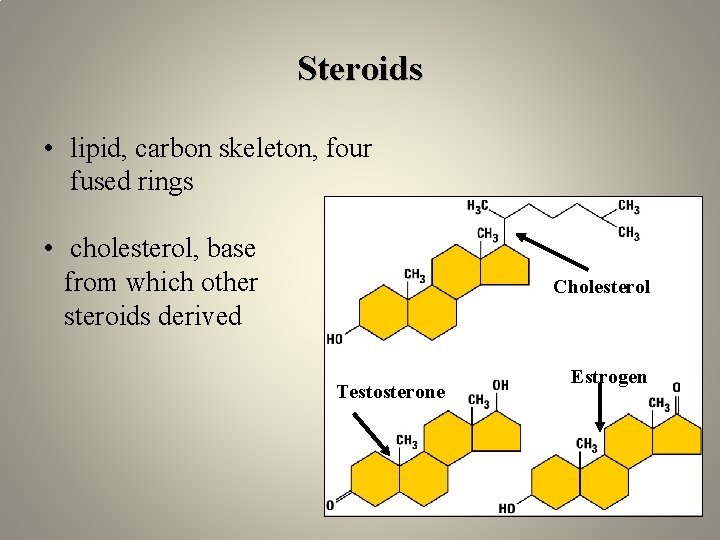
Steroids • lipid, carbon skeleton, four fused rings • cholesterol, base from which other steroids derived Cholesterol Testosterone Estrogen
Steroids • cholesterol reduces membrane fluidity by reducing phospholipid movement • androgens and estrogens affect sexual development and function • glucocorticoids influence metabolism and inflammatory reactions http: //mrlab. frsc. tsukuba. ac. jp/human_embryos/human_embryo. html
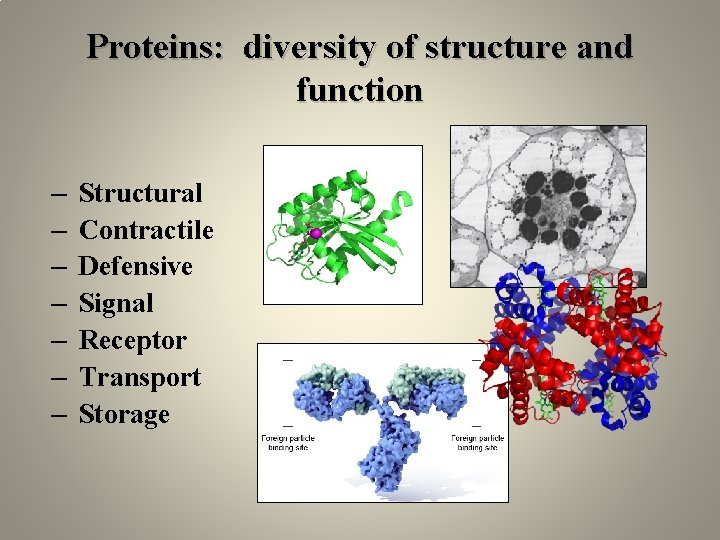
Proteins: diversity of structure and function – – – – Structural Contractile Defensive Signal Receptor Transport Storage
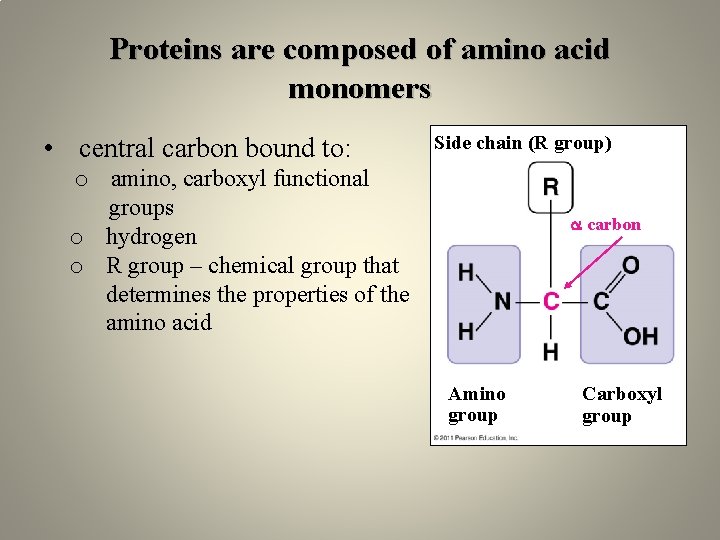
Proteins are composed of amino acid monomers • central carbon bound to: Side chain (R group) o amino, carboxyl functional groups o hydrogen o R group – chemical group that determines the properties of the amino acid carbon Amino group Carboxyl group
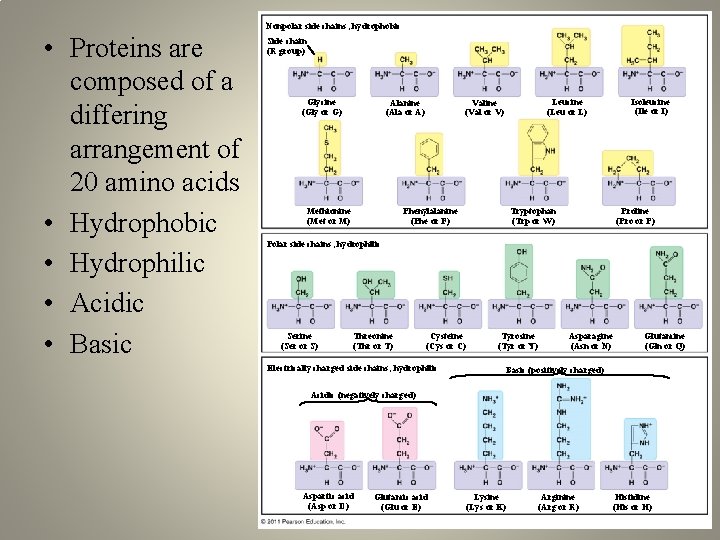
Nonpolar side chains; hydrophobic • Proteins are composed of a differing arrangement of 20 amino acids • Hydrophobic • Hydrophilic • Acidic • Basic Side chain (R group) Glycine (Gly or G) Alanine (Ala or A) Methionine (Met or M) Isoleucine (Ile or I) Leucine (Leu or L) Valine (Val or V) Phenylalanine (Phe or F) Tryptophan (Trp or W) Proline (Pro or P) Polar side chains; hydrophilic Serine (Ser or S) Threonine (Thr or T) Cysteine (Cys or C) Electrically charged side chains; hydrophilic Tyrosine (Tyr or Y) Asparagine (Asn or N) Glutamine (Gln or Q) Basic (positively charged) Acidic (negatively charged) Aspartic acid (Asp or D) Glutamic acid (Glu or E) Lysine (Lys or K) Arginine (Arg or R) Histidine (His or H)
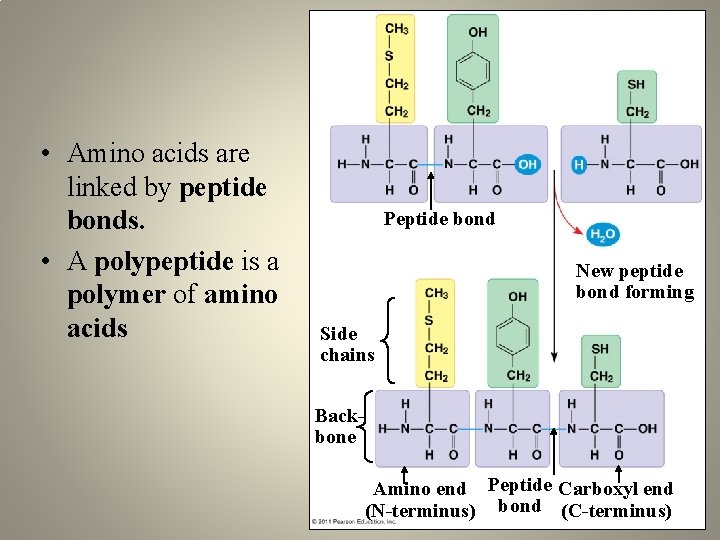
• Amino acids are linked by peptide bonds. • A polypeptide is a polymer of amino acids Peptide bond New peptide bond forming Side chains Backbone Amino end Peptide Carboxyl end (N-terminus) bond (C-terminus)
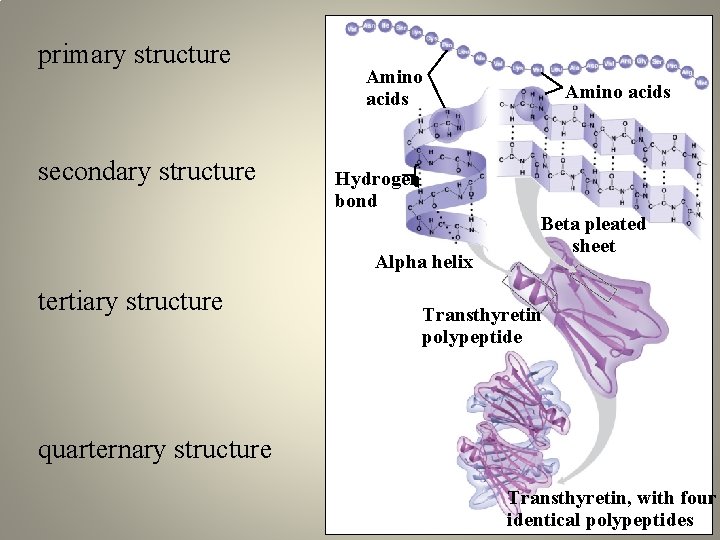
primary structure secondary structure Amino acids Hydrogen bond Alpha helix tertiary structure Amino acids Beta pleated sheet Transthyretin polypeptide quarternary structure Transthyretin, with four identical polypeptides
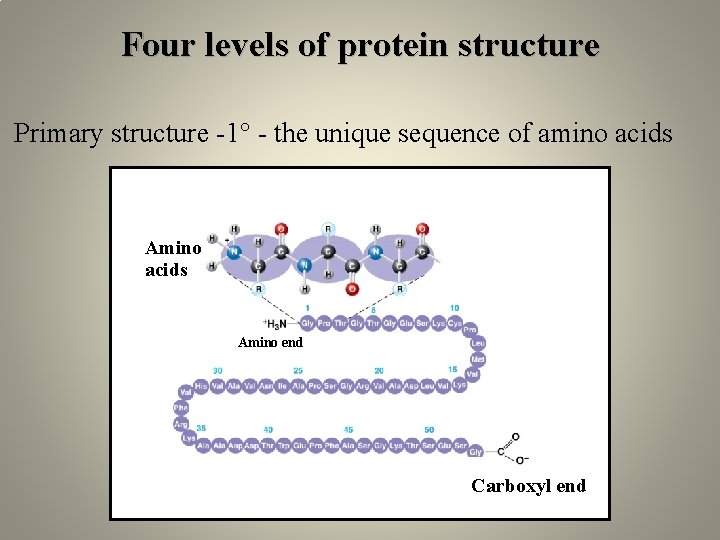
Four levels of protein structure Primary structure -1° - the unique sequence of amino acids Amino end Carboxyl end
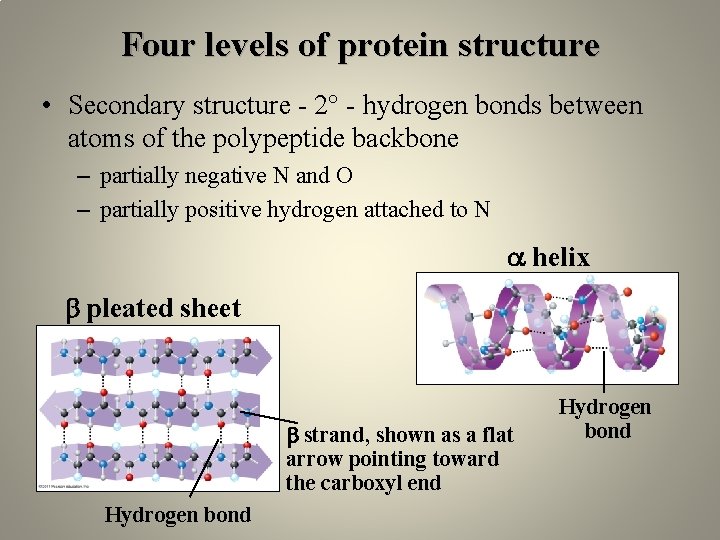
Four levels of protein structure • Secondary structure - 2° - hydrogen bonds between atoms of the polypeptide backbone – partially negative N and O – partially positive hydrogen attached to N helix pleated sheet strand, shown as a flat arrow pointing toward the carboxyl end Hydrogen bond
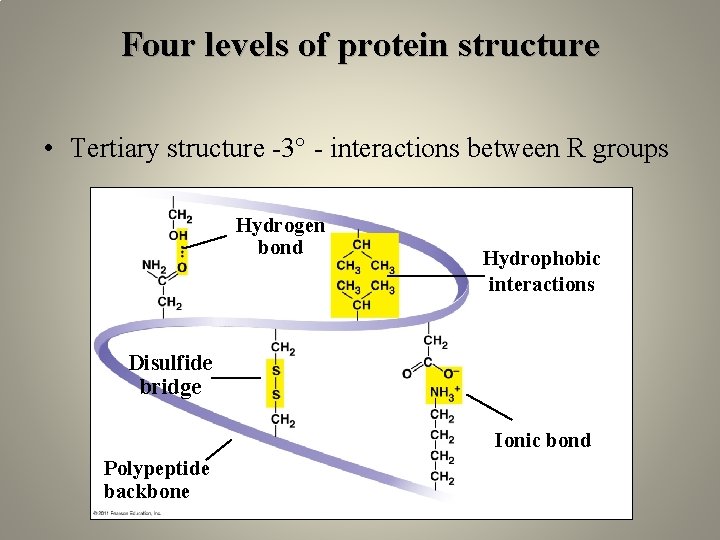
Four levels of protein structure • Tertiary structure -3° - interactions between R groups Hydrogen bond Hydrophobic interactions Disulfide bridge Ionic bond Polypeptide backbone
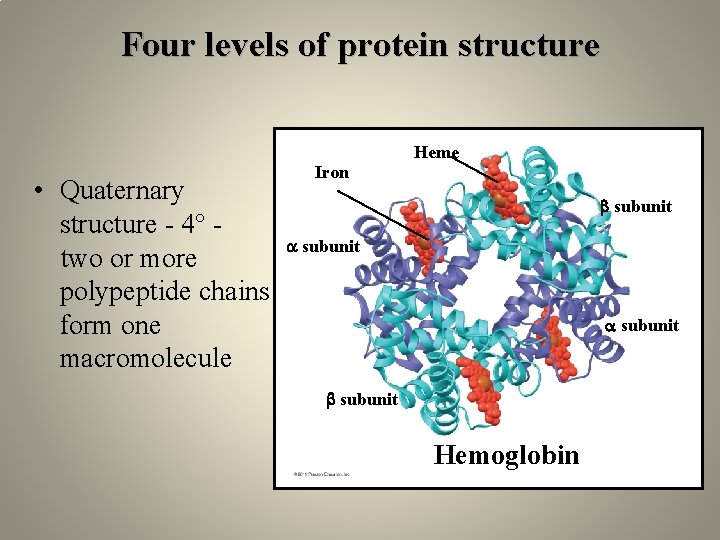
Four levels of protein structure Heme • Quaternary structure - 4° two or more polypeptide chains form one macromolecule Iron subunit Hemoglobin
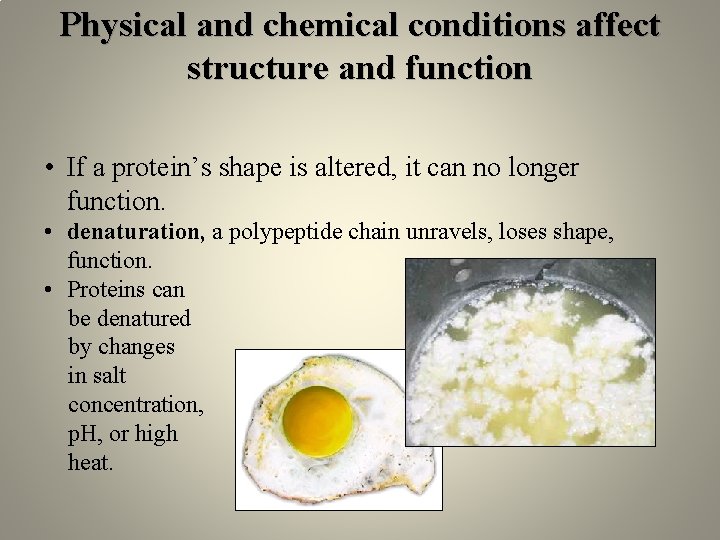
Physical and chemical conditions affect structure and function • If a protein’s shape is altered, it can no longer function. • denaturation, a polypeptide chain unravels, loses shape, function. • Proteins can be denatured by changes in salt concentration, p. H, or high heat.
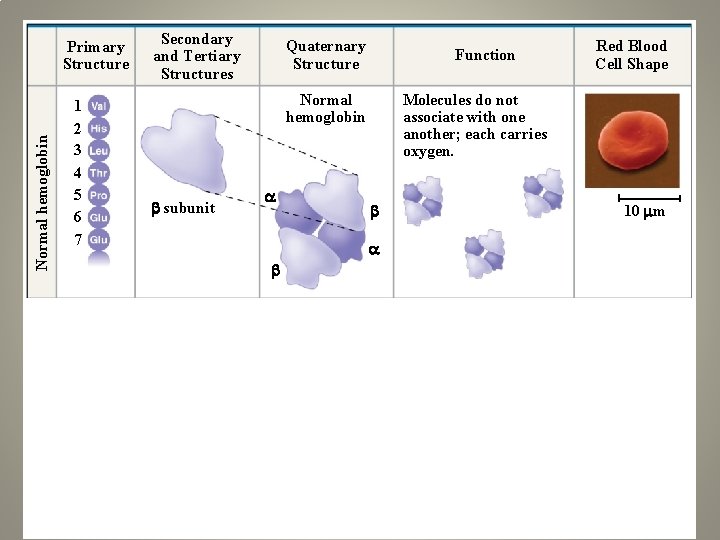
Sickle-cell hemoglobin Normal hemoglobin Primary Structure 1 2 3 4 5 6 7 Secondary and Tertiary Structures Quaternary Structure Function Molecules do not associate with one another; each carries oxygen. Normal hemoglobin subunit Red Blood Cell Shape 10 m 1 2 3 4 5 6 7 Exposed hydrophobic region Sickle-cell hemoglobin subunit Molecules crystallize into a fiber; capacity to carry oxygen is reduced. 10 m
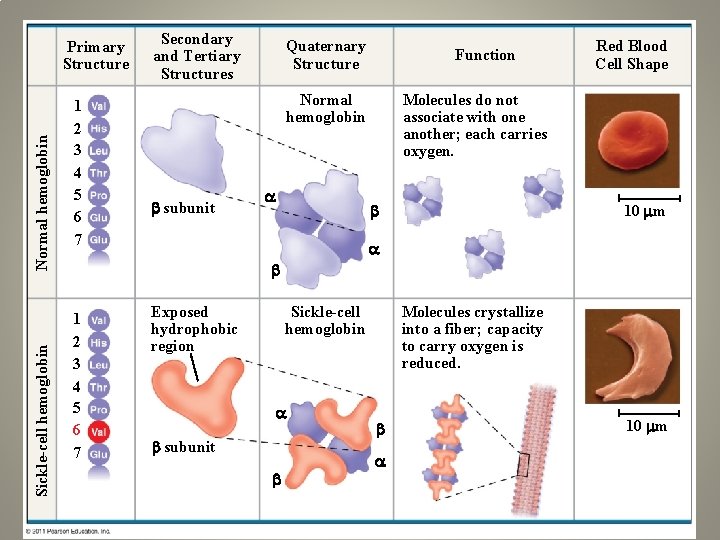
Sickle-cell hemoglobin Normal hemoglobin Primary Structure 1 2 3 4 5 6 7 Secondary and Tertiary Structures Quaternary Structure Function Molecules do not associate with one another; each carries oxygen. Normal hemoglobin subunit Red Blood Cell Shape 10 m 1 2 3 4 5 6 7 Exposed hydrophobic region Sickle-cell hemoglobin subunit Molecules crystallize into a fiber; capacity to carry oxygen is reduced. 10 m
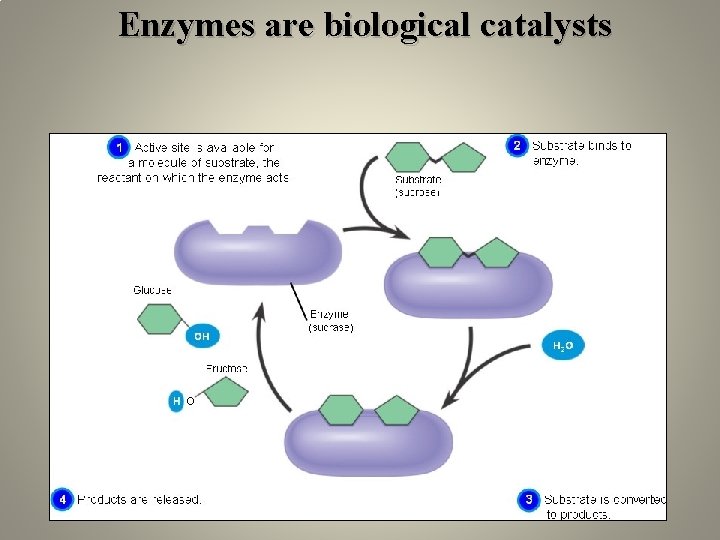
Enzymes are biological catalysts
Nucleic Acids • store, transmit and help express hereditary material • Two types of nucleic acids – Deoxyribonucleic acid (DNA) – Ribonucleic acid (RNA) • DNA provides directions for its own replication • DNA directs synthesis of messenger RNA (m. RNA) and, through m. RNA, controls protein synthesis
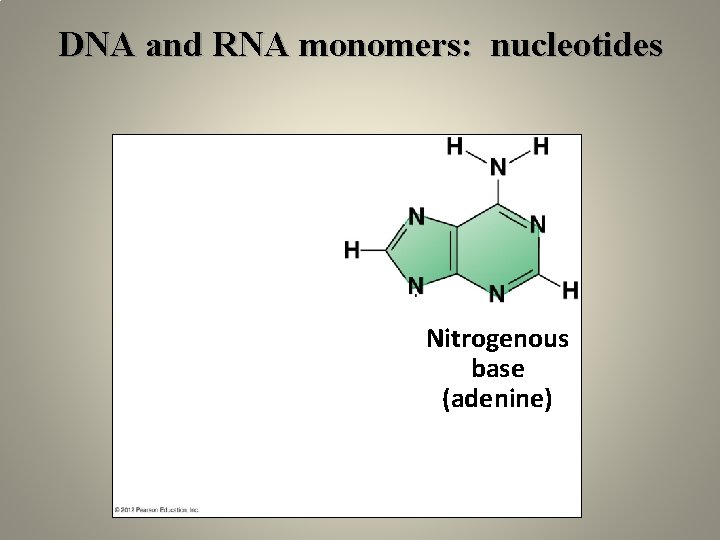
DNA and RNA monomers: nucleotides Nitrogenous base (adenine) Phosphate group Sugar
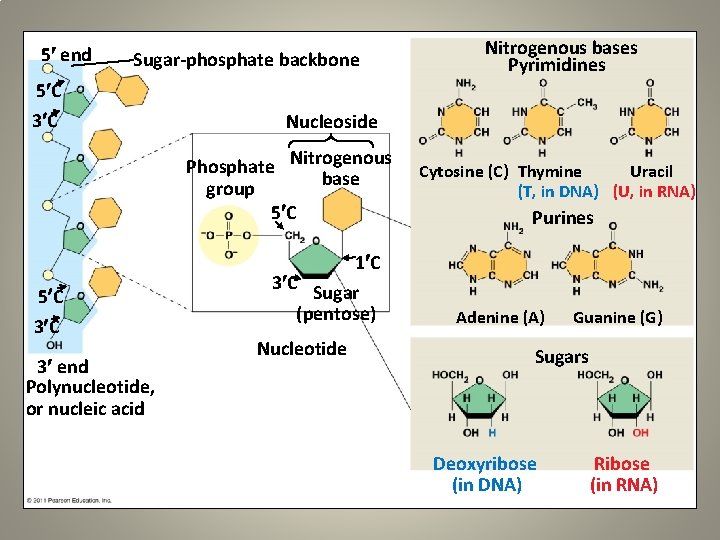
5 end Sugar-phosphate backbone 5 C 3 C Nitrogenous bases Pyrimidines Nucleoside Phosphate Nitrogenous base group 5 C Cytosine (C) Thymine Uracil (T, in DNA) (U, in RNA) Purines 1 C 5 C 3 end Polynucleotide, or nucleic acid 3 C Sugar (pentose) Nucleotide Adenine (A) Guanine (G) Sugars Deoxyribose (in DNA) Ribose (in RNA)
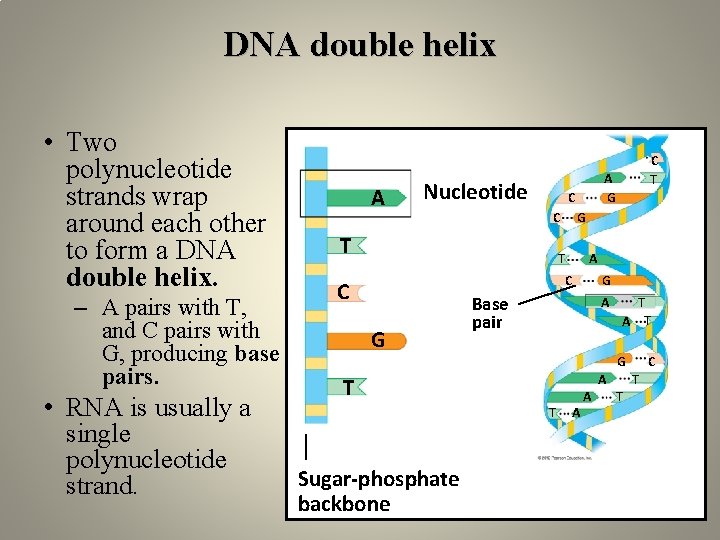
DNA double helix • Two polynucleotide strands wrap around each other to form a DNA double helix. – A pairs with T, and C pairs with G, producing base pairs. • RNA is usually a single polynucleotide strand. A Nucleotide A G C C T G T A C C G G Base pair A T G T T Sugar-phosphate backbone C T A A A T C T
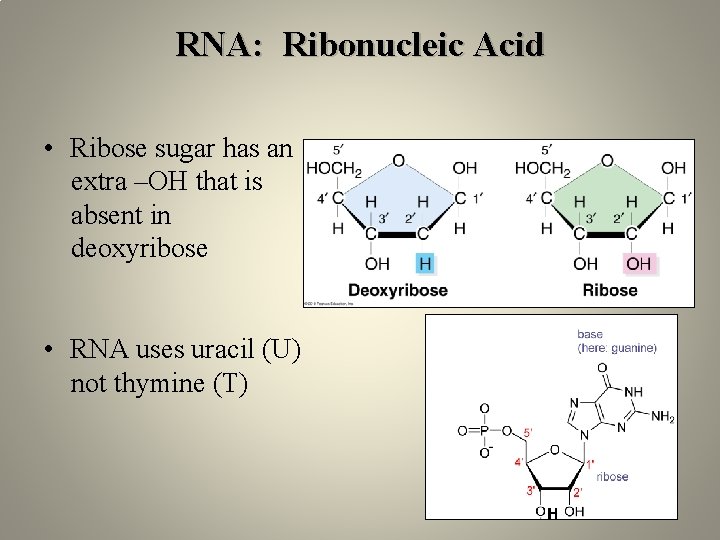
RNA: Ribonucleic Acid • Ribose sugar has an extra –OH that is absent in deoxyribose • RNA uses uracil (U) not thymine (T) H
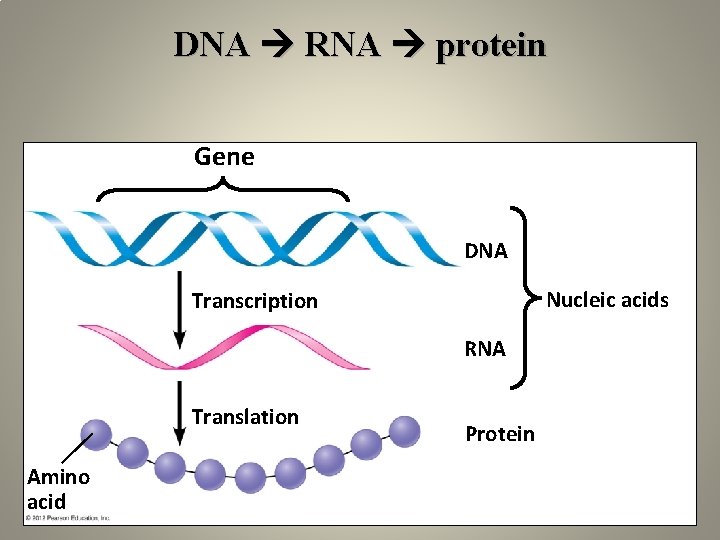
DNA RNA protein Gene DNA Nucleic acids Transcription RNA Translation Amino acid Protein
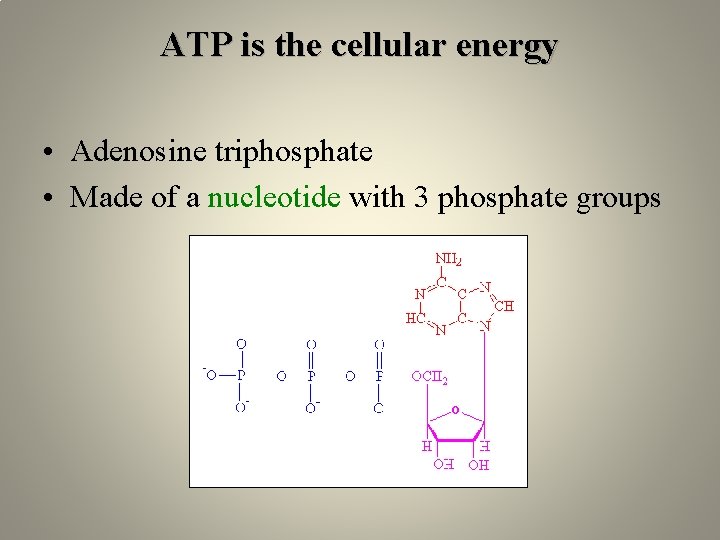
ATP is the cellular energy • Adenosine triphosphate • Made of a nucleotide with 3 phosphate groups
- Slides: 44