Bright Line Spectra Thomas V Danahy Nanuet Senior

Bright Line Spectra Thomas V. Danahy Nanuet Senior High School

Ground State vs. Excited State Ground State – all electrons are in the lowest possible energy levels (normal) ex. 2 – 8 – 18 – 32 Excited State – if given additional energy, electrons will “jump up” to higher energy levels, temporarily. Excited State ex. 2 – 5 – 2 Ground State ex. 2 – 7
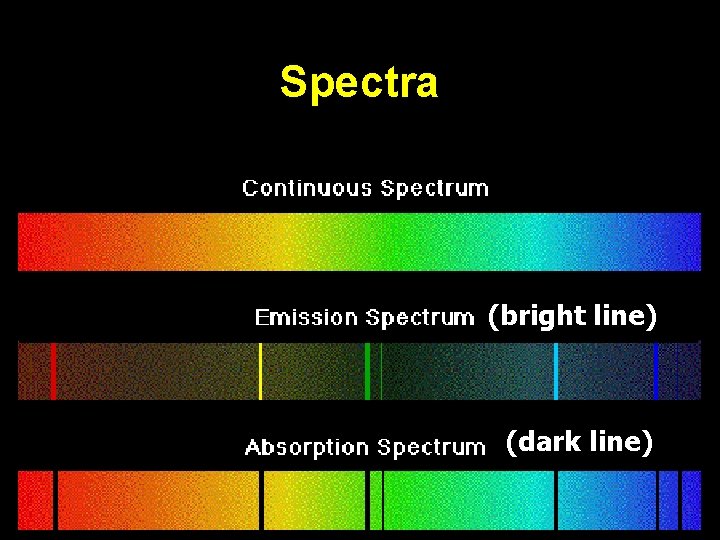
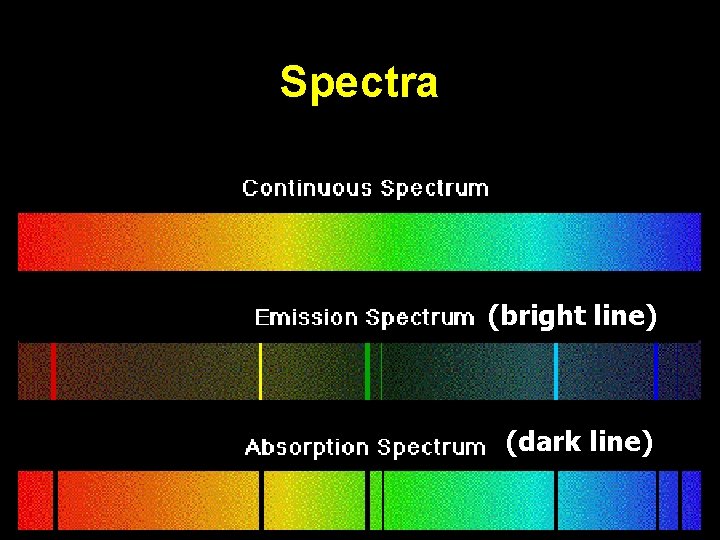
Spectra (bright line) (dark line)

Bright Line = Emission Spectra How does this happen? “Excited electrons” at higher energy levels will eventually release the extra energy and “fall back down” to ground state conditions. During the “fall back”, energy is released as Visible Light Energy. Wavelengths = Energy = Color Bands
Bright Line Spectra
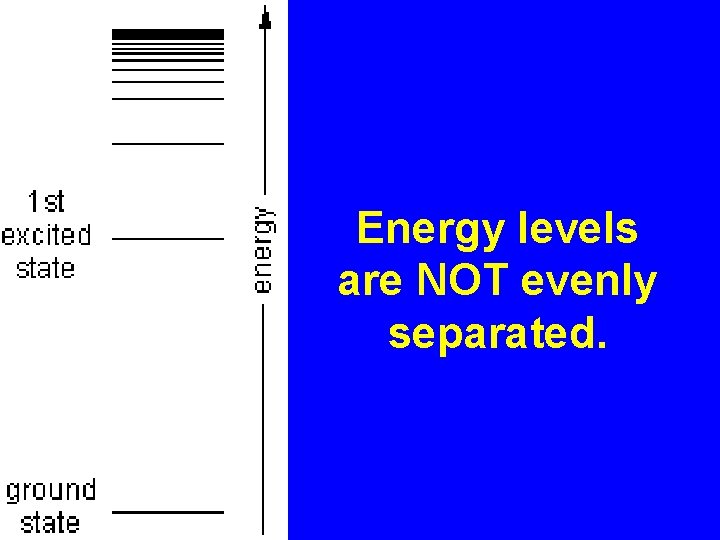
Energy levels are NOT evenly separated.
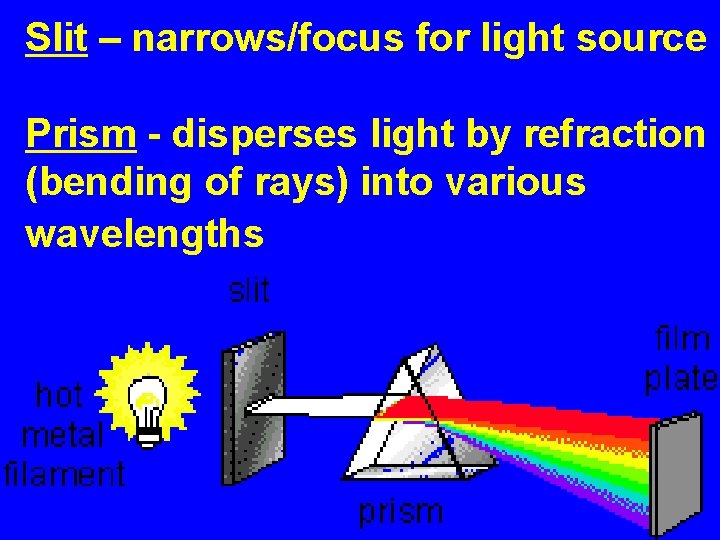
Slit – narrows/focus for light source Prism - disperses light by refraction (bending of rays) into various wavelengths
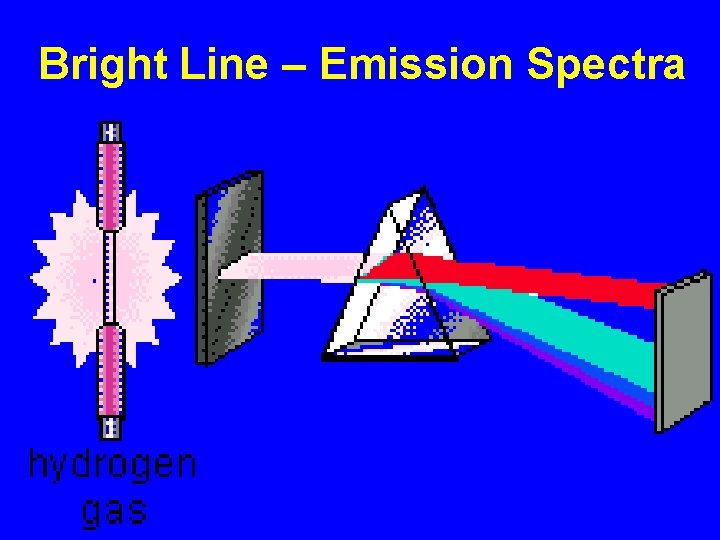
Bright Line – Emission Spectra
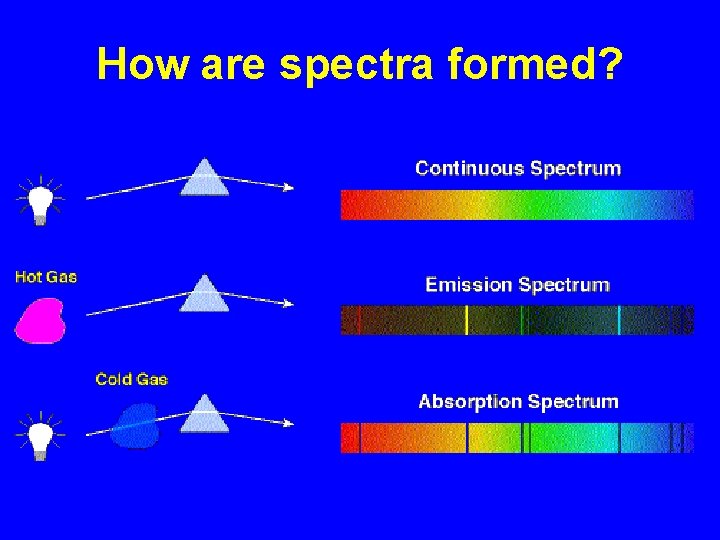
How are spectra formed?

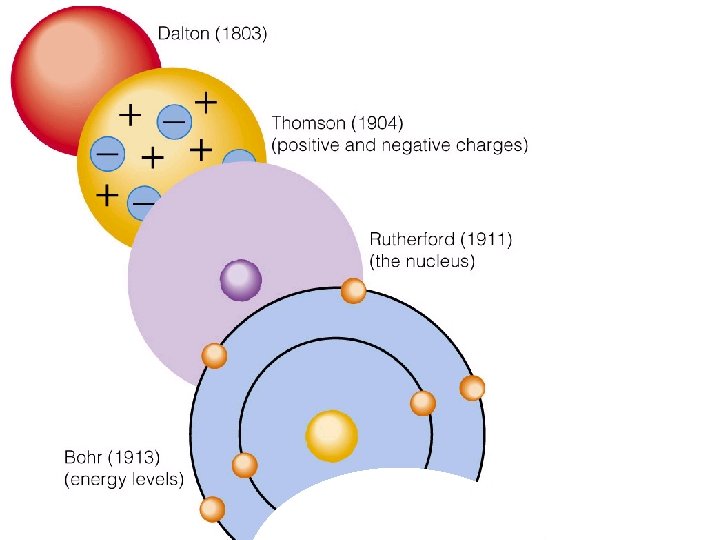
Niels Bohr 1913 Danish chemist Bohr model of atom: http: //www. chemeng. uiuc. edu/~alkgrp/mo/gk 12/qu antum/
Bright Line Spectra

Each element has a specific electron configuration and a corresponding emission spectrum. Emission (bright line) spectrum can be used to identify (“fingerprint”) each element. http: //www. colorado. edu/UCB/Academic. Affairs/Arts. Sci ences/physics/Physics. Initiative/Physics 2000/applets/a 2. html

So Dude, check out the colors! Do the spectra lab.
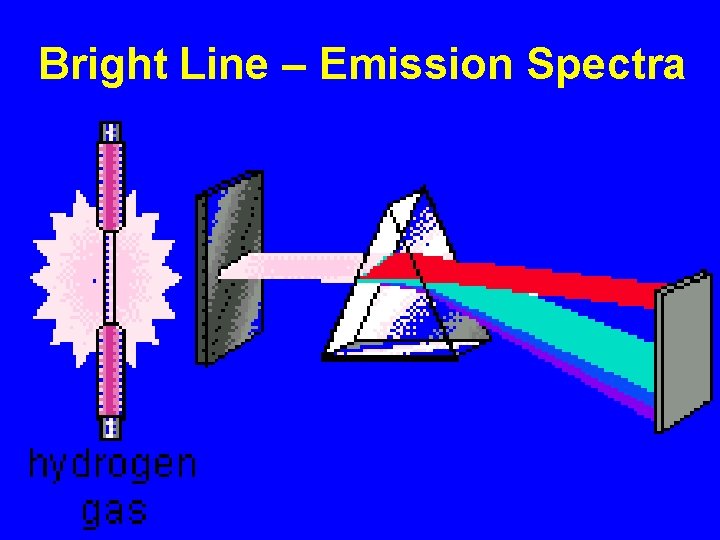
Bright Line – Emission Spectra
Spectra (bright line) (dark line)

Flame Tests - burn metal salts in a flame and observe/record the color - compare colors to known standards for metals - spectrascope not required
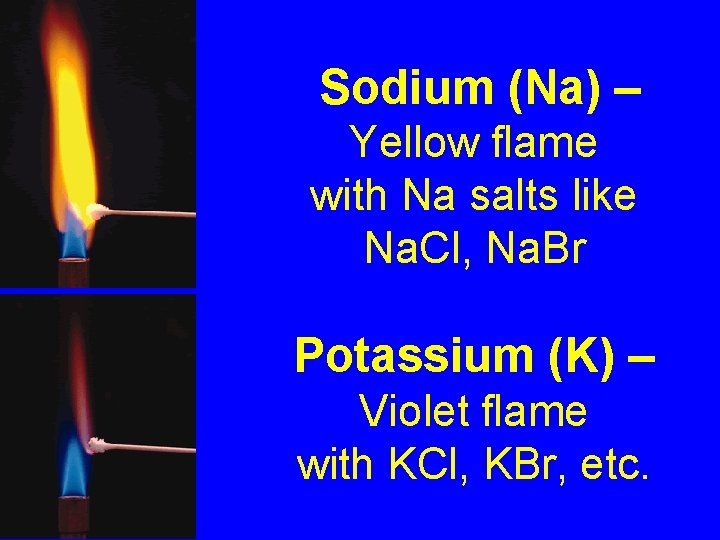
Sodium (Na) – Yellow flame with Na salts like Na. Cl, Na. Br Potassium (K) – Violet flame with KCl, KBr, etc.

De. Broglie – Wave Function - complete wavelength multiples needed for stable circular orbits. (after entering webpage, scroll down & click of De. Broglie demo) http: //www. colorado. edu/physics/2000/quantumzone/debroglie. html
- Slides: 18