Bridging the Skills Gap for the Pharmaceutical Industry

Bridging the Skills Gap for the Pharmaceutical Industry in South Africa Human Capital Outlook Implications for Skills Development in the Pharmaceutical Sector Johannesburg May 27 2015 By Dr Skhumbuzo Ngozwana

Disclaimer Any views or opinions expressed herein are solely those of the author and do not necessarily represent those of any company. www. metanoia. co. za

“A dwarf standing on the shoulders of a giant can see further than the giant” Sir Isaac Newton – private correspondence to Robert Hooke

Outline v Framing the Debate for Skills Development v Challenges & Barriers of SA / Africa Based Pharmaceutical Manufacturers v Manufacturing processes & skills requirements v Key research findings from the DTI Study v Recommendations q. Framework for Policy Alignment q. Revise & Review curriculum q. Facilitate international partnerships & collaboration www. metanoia. co. za 4

Framing the Debate for the Imperative for Skills Development

High Disease Burden (current) v 25% of the global disease burden q 75% of the global HIV/AIDS pandemic q 90% of the malaria cases and deaths q 9 countries (excluding North Africa) among the 22 countries with the highest TB burden in the world. q. MDR-TB and XDR-TB rated among the highest in the world. q. Significant child mortality – diarrhoeal, measles, URTI q. An emerging CD epidemic Source: Mckinsey & Co, WHO, ADB, Stop. TB www. metanoia. co. za 6
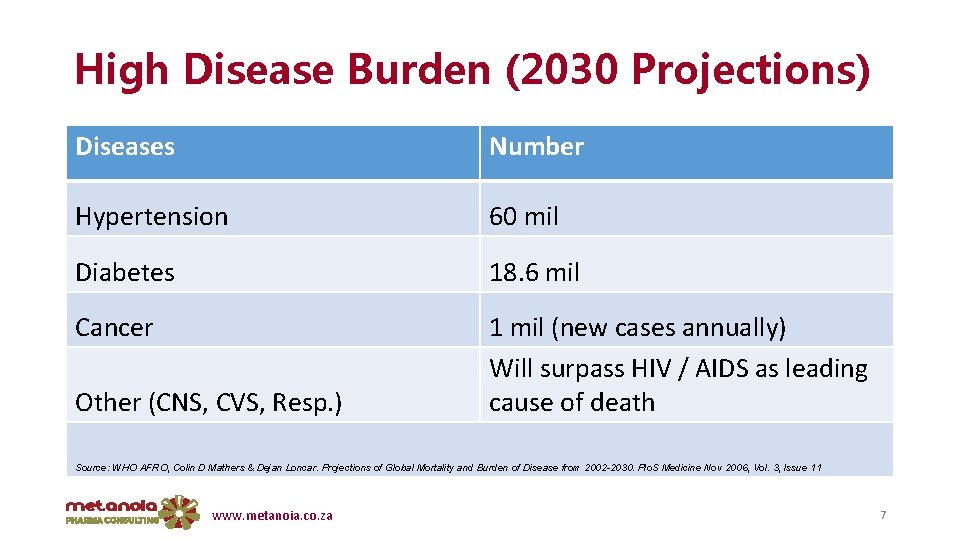
High Disease Burden (2030 Projections) Diseases Number Hypertension 60 mil Diabetes 18. 6 mil Cancer 1 mil (new cases annually) Will surpass HIV / AIDS as leading cause of death Other (CNS, CVS, Resp. ) Source: WHO AFRO, Colin D Mathers & Dejan Loncar. Projections of Global Mortality and Burden of Disease from 2002 -2030. Plo. S Medicine Nov 2006, Vol. 3, Issue 11 www. metanoia. co. za 7

Challenges of SA / Africa Based Pharmaceutical Manufacturers

SA / African Pharma at a Glance… v Estimated at around $ 20 billion (RSA ~4. 2 b) q IMS Health (Global Market Prognosis) 2010 Africa at 13, 879 Bn USD & but expected to reach $30 billion by 2016 q Growth has surpassed that initial projection & IMS predicts 45 bn by 2020 v Imports: +/- 95% of API & +/- 70% of FF v Growing in double digits v Growing focus on developing the sector across the continent (PMPA, REC Plans, Country plans) Source: IMS Health www. metanoia. co. za 9
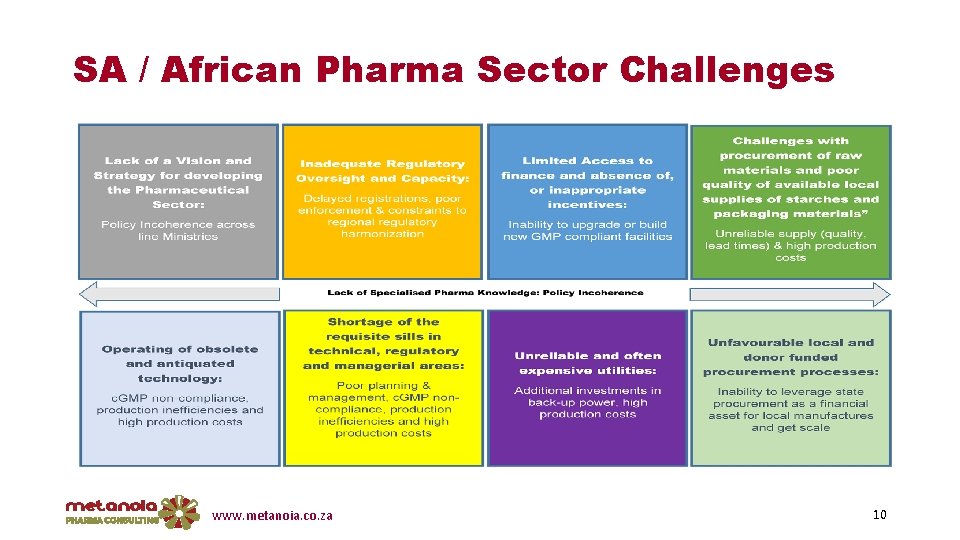
SA / African Pharma Sector Challenges www. metanoia. co. za 10

Critical Lack of Skills v Drug development (reverse engineering, NCE’s, novel combinations and general formulation development) v Drug testing (quality control and assurance) v Regulatory medicine and supply chain regulation (pharmaco-vigilance, GWP, GDP, DLP, GCP) v Drug manufacturing (GMP, Lean manufacturing, plant operations and maintenance) v Pharmaceutical management (production planning, supply chain management, entrepreneurship, governance, commercial law, operations, financial management, sales and marketing efforts etc. ) v Lack of pharmaceutical policy expertise to address policy incoherence, craft sector strategies etc. www. metanoia. co. za

The impact of the lack of critical Skills v Inability to adopt “Leap-frogging” technologies to catch up (NSI) v Weak regulatory systems – inability to regulate the pharmaceutical industry across the entire value chain properly & registration delays v Poor quality dossiers leading to registration delays v Inability to formulate own products resulting in limited portfolios v Lack of bioequivalence expertise and centres v Poor quality production and the attendant problem of sub-standard medicines v Inefficient production with lots of wastage and a higher cost base v Poor overall business management v Total reliance on others for the supply of all our needs www. metanoia. co. za

Manufacturing Processes & Skills Requirements

Pharmaceutical Production Process Flow www. metanoia. co. za 14

Biotech manufacturing process Flow Tissue collection or bacterial fermentation or cell culture Tissue extraction Cell separation Cell culture fluid clarification Cell membrane extraction Bacterial capsule extraction Cell Lysis www. metanoia. co. za Cell purification (downstream purification) Viral clearance & aseptic filtration for final vaccine 15

Functions & disciplines Functions Disciplines Process Chemistry Biosynthesis Process Microbiology Process Engineering Process Design Process Chemistry Process Microbiology Analytical chemistry R&D Technology Transfer Regulatory Affairs Manufacturing and Controls Technology Transfer Validation Process Engineering Equipment Validation Engineering Process Validation Cleaning Validation Computerised Systems Validation Process Development Process Design www. metanoia. co. za 16
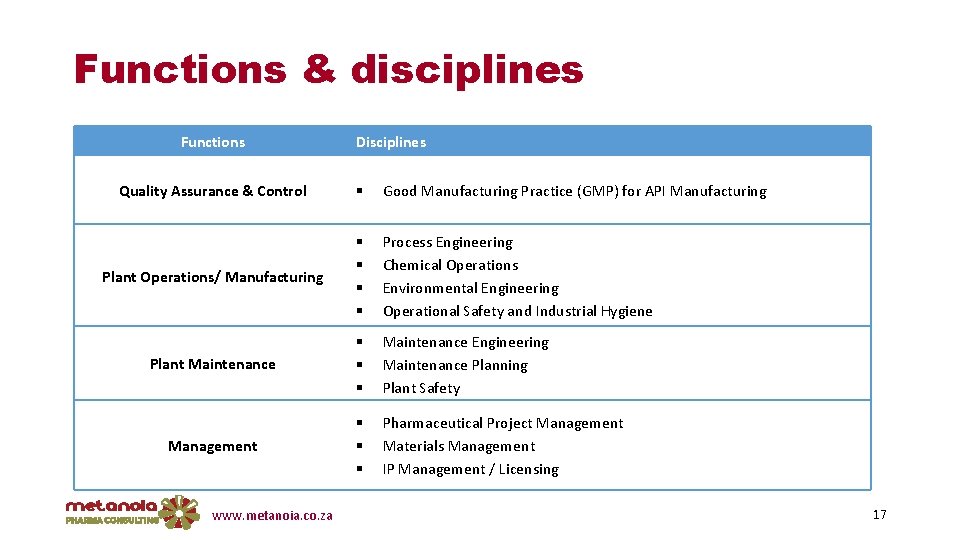
Functions & disciplines Functions Disciplines Quality Assurance & Control Good Manufacturing Practice (GMP) for API Manufacturing Plant Operations/ Manufacturing Process Engineering Chemical Operations Environmental Engineering Operational Safety and Industrial Hygiene Plant Maintenance Engineering Maintenance Planning Plant Safety Management Pharmaceutical Project Management Materials Management IP Management / Licensing www. metanoia. co. za 17

Skills & Specialisations Field of study specialisations Chemistry Biological sciences Life sciences www. metanoia. co. za API Bio tech Analytical √ √ organic √ synthetic √ Microbiology √ √ molecular biology √ Biochemistry √ Pharmacy (production & regulatory) √ √ Pharmaceutical science √ √ Virology √ Biotechnology √ 18
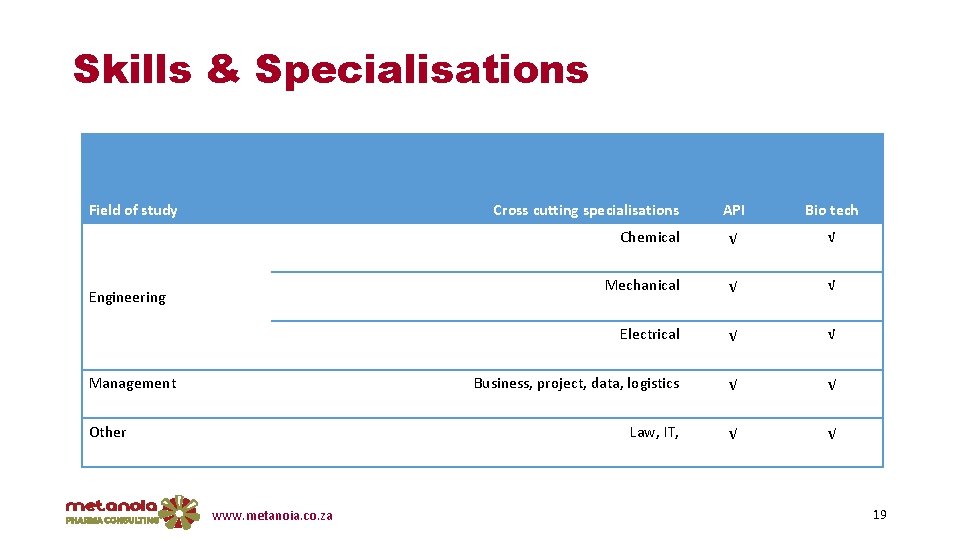
Skills & Specialisations Field of study Cross cutting specialisations Engineering Management Other www. metanoia. co. za API Bio tech Chemical √ √ Mechanical √ √ Electrical √ √ Business, project, data, logistics √ √ Law, IT, √ √ 19

Key Research Findings

Key findings v No clear pharmaceutical industry strategy: q. No shared vision & direction q. Superficial understanding of the industry v HET offering q. Generic science qualifications (academic-oriented) o Chemistry offering inadequate (analytical, synthetic, organic offered under general chemistry) o No regulatory, production, QC training in pharmacy curriculum o No industrial applicability www. metanoia. co. za 21

Key findings… 2 v. HET offering … q. Strong on theory, weak on industrial practice q“ >3 years of OTJ training to become productive in the pharmaceutical manufacturing environment“ v. Output quantities: no critical mass of skills base www. metanoia. co. za 22
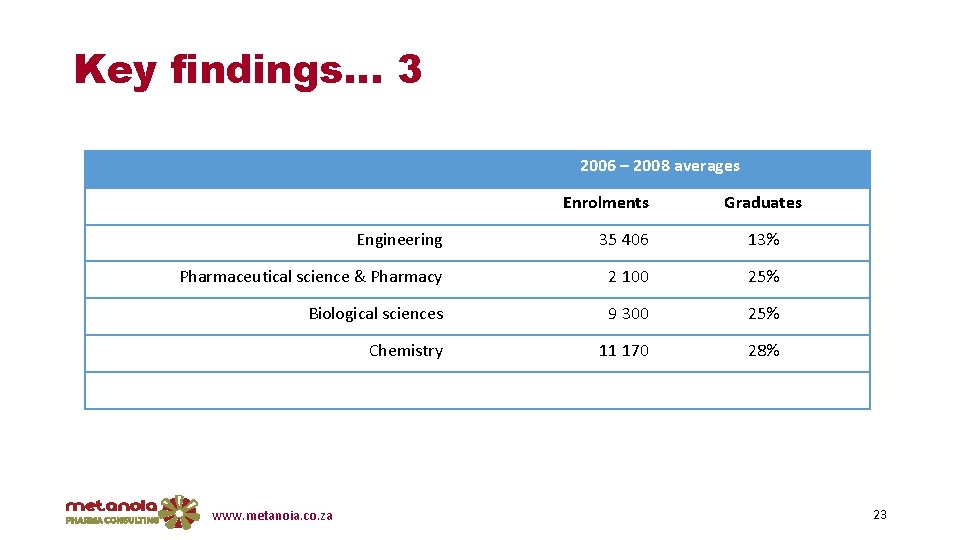
Key findings… 3 2006 – 2008 averages Enrolments Graduates Engineering 35 406 13% Pharmaceutical science & Pharmacy 2 100 25% Biological sciences 9 300 25% Chemistry 11 170 28% www. metanoia. co. za 23
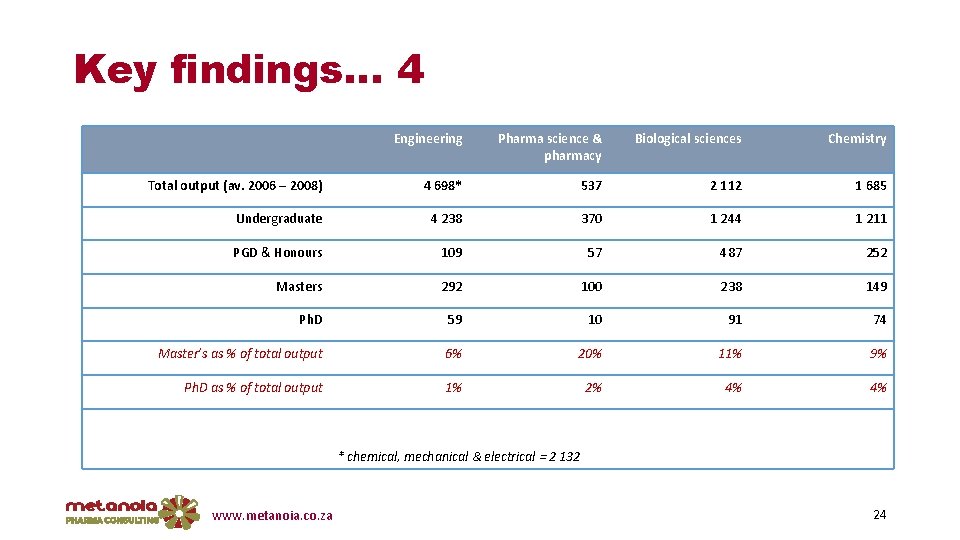
Key findings… 4 Engineering Pharma science & pharmacy Biological sciences Chemistry Total output (av. 2006 – 2008) 4 698* 537 2 112 1 685 Undergraduate 4 238 370 1 244 1 211 PGD & Honours 109 57 487 252 Masters 292 100 238 149 Ph. D 59 10 91 74 Master’s as % of total output 6% 20% 11% 9% Ph. D as % of total output 1% 2% 4% 4% * chemical, mechanical & electrical = 2 132 www. metanoia. co. za 24

Critical success ingredients Industry -collaboration -R & D -Training HEIs - Training SET Infrastructure Research Council Success! - R&D - Tech transfer policy & regulatory framework Funding & incentives Education - Basic universal - Postgraduate www. metanoia. co. za 25

Recommendations

Recommendations: 1. Create a Framework for Policy Alignment – SA Core Documents
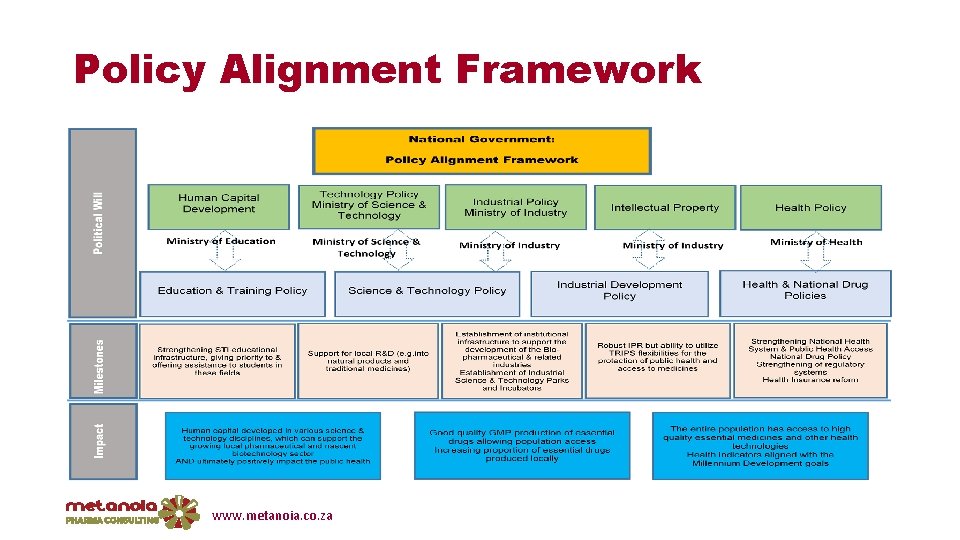
Policy Alignment Framework www. metanoia. co. za

National Development Plan 2050 v The plan articulates the vision for healthcare in South Africa as follows: q “We envisage that in 2030, South Africa has a life expectancy of at least 70 years for men and women…, the quadruple burden of disease have been markedly reduced compared to the two previous decades”. v The NDP also aspires to: q Ensure that the population of under 20’s free of HIV / AIDS increases q Progressively improve TB prevention and cure by 2030 q Reduce the prevalence of non-communicable chronic diseases by 28% by 2030 National Development Plan: Vision for 2030. v Calls for the government to invest in technological revolutions of the 21 st century - specifically highlighting biotechnology and nanotechnology www. metanoia. co. za

Dept. of Science and Technology’s Bio-economy Strategy v The key Strategic priorities as outlined in the Bioeconomy strategy include the following: qto develop improved therapeutic and drug delivery systems to address priority diseases qto develop new and improved vaccines and biologics qdevelop improved diagnostics qdevelop improved medical devices qstrengthen clinical research and development capabilities, and qestablish pharmaceutical manufacturing in the country v Specifically, the Bioeconomy strategy calls for the prioritization of drug development and proposes that this be pursued via publicprivate partnerships (PPPs). www. metanoia. co. za

Department of Trade and Industry’s Industrial Policy Action Plan (IPAP) v IPAP identifies a number of priority sectors to be supported for development in order to address South Africa’s health challenges and dependence on imported products. IPAP specific areas identified for extraordinary government support are: qproduction of ARV API’s qproduction of vaccines qproduction of biological medicines qproduction of diagnostics and medical devices www. metanoia. co. za

National Dept. of Health’s Strategic Plan v Mission q. To improve health status through prevention of illness, disease and promotion of healthy lifestyles, and to consistently improve the healthcare delivery system by focusing on access, equity, efficiency, quality and sustainability v Core focus on improving access to affordable, safe, efficacious medicines, including to newer therapies v Has five key priority areas: HIV / AIDS, TB, Cancer, Maternal & Child Health and Diabetes v Has entered into a number of PPP / PPM projects q. Biovac Institute – revive the declining vaccine production in RSA & supply the EPI q. Multiple PPP’s in the hospital sector www. metanoia. co. za

Recommendations: 2. Develop a National Vision & Strategy for the Sector

Recommendations v Development of a National Vision & sector strategy q. Informed by extensive sector research & consultations q. Clear vision & growth path for the industry, aligned with key gov’t policies (Trade & industry, DST, Health, Mo. E, Finance, etc. ) v Investigate the role that the NPC can play in skills planning & development v Adopt a cluster approach v The Diaspora effect…. . reverse brain gain (incentives, conducive environment etc. . ) www. metanoia. co. za 34
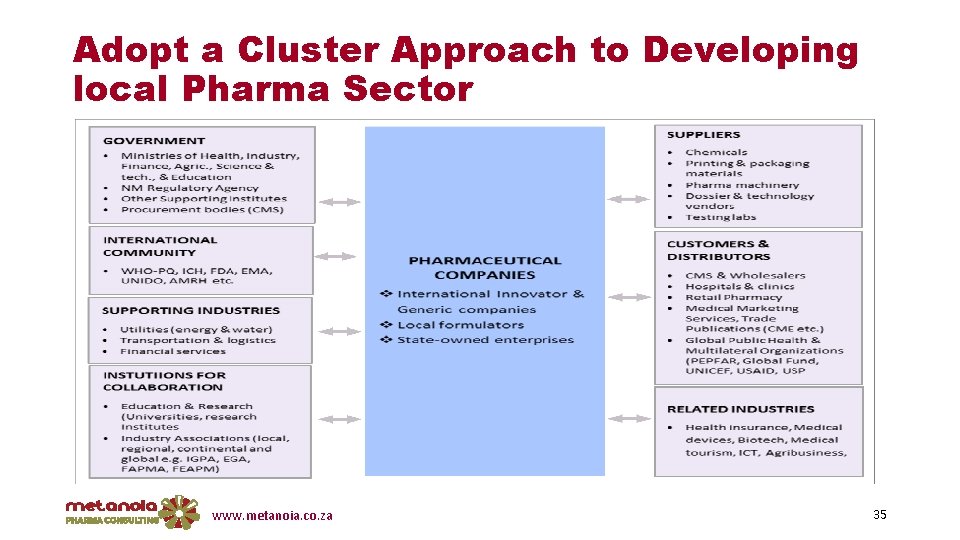
Adopt a Cluster Approach to Developing local Pharma Sector www. metanoia. co. za 35

Recommendations: 2. Review & Revise Training curriculum

Strengthen STI related training v Strengthen foundational education in STI related fields v Review & reorientation of Pharmacy & Science, engineering curricula v Strengthen & Incentivize Industry-Academia collaborations v Tap into the African Diaspora v Establishment of specialised pharmaceutical training / R&D institutes q India NIPER / NCL / IICT, Nigeria’s NIPRD q RSI SA q Tanzania St Luke’s, MUHAS q US Stephens Institute of technology www. metanoia. co. za 37

Curriculum Review v Formal training & Short courses / Seminars in: q. Drug development (new chemical entities, novel combinations like FDC’s, paediatric, delivery systems; and general formulation development) q. Drug testing (quality control and assurance) q. Drug approval (regulatory medicine) and supply chain regulation (PMS/ pharmaco-vigilance, Good Manufacturing Practice, Good Wholesaling Practice, Good Distribution Practice, Good Laboratory Practice, Good Clinical Practice) q. Drug manufacturing (GMP, TPM, Lean manufacturing, plant operations and maintenance) q. Pharmaceutical management (production planning, supply chain management, entrepreneurship, governance, commercial law, business development, operations, financial management, pharmaceutical sales and marketing etc. ) www. metanoia. co. za

Recommendations: 3. Create International Partnerships & Strengthen Collaboration

St Lukes Foundation v St Luke’s Foundation offers an Industrial Pharmacy Advanced Training postgraduate program (with Purdue and Howard universities in the US). q. Drug development and Regulatory quality Compliance q. Drug Manufacturing Process (GMP) q. Regulatory Documents and Generic Drug Approval submissions q. Drug Discovery. v St Lukes also offers basic, technicians certificate, technician’s certificate and a diploma in pharmaceutical science to pharmaceutical operators. www. metanoia. co. za

AMRH & ANDI v AMRH q. Continental Technical Working Group on Regulatory Capacity Development (TWGRCD) operational since 2012 q 10 Regional Centres of Regulatory Excellence (RCOREs) q. Pool of regulatory experts since Oct 2013 q. TWG embarked on the development of a harmonised curricula for regulatory capacity development v ANDI q 32 Centers of Excellence across the continent (Drug Discovery, Diagnostics, Devices Appropriate for our setting) q. Ebola (Genome work & Rapid Diagnostics) www. metanoia. co. za

Muhimbili University of the Health and Allied Sciences v MUHAS offers practical training for pharmaceutical professionals in the areas of : q. Formulation development q. GMP q. Process scale-up q. Stability and analytical testing q. Tableting and coating q. Quality control of medicines q. Sustained release formulations www. metanoia. co. za

International Society for Pharmaceutical Engineers v International Society for Pharmaceutical Engineers (ISPE) membership includes engineers, microbiologists, chemists, QA/QC, production, process development, pharmacists, regulatory and training personnel, academia, and suppliers; qexists for the sole reason of improving efficiency and best practices, q. ISPE offers various training events across the world, and on-site at companies, where leading industry experts impart knowledge and skills to pharmaceutical teams from around the world. q. ISPE also offers an extensive curriculum of online courses across various manufacturing issues. A selection of some of the leading programs offered by ISPE includes cleaning, manufacturing facilities, GMP, HVAC, manufacturing (QRM, clinical trials, QA/QC, Q 7 A etc. ) and validation www. metanoia. co. za

United States Pharmacopeial Convention v USP’s Promoting the Quality of Medicines (PQM) initiative works with regulators to build the capacity in medicine regulation to ensure that there adequate quality assurance measures in place. v USP PQM offers National Medicines Regulatory Authorities (NMRAs) training and technical assistance to formulate medicine regulation; strengthen regulation, and to introduce good regulatory practices and international quality standards. v USP Global Health Program - Centre for Pharmaceutical Advancement and Training (Ce. PAT), in Accra, Ghana. q Ce. PAT will become a centre of excellence for Sub- Saharan Africa (SSA) - build the human resources needed to properly control the quality of pharmaceuticals in the region and to curb the scourge of counterfeits and sub-standard drugs. q This centre is intended to benefit regulators, medicines quality control laboratories, manufacturers, and donor agencies and procurement organisations. v USP, in collaboration with the WHO and the Global Drug Facility, also offers WHOprequalification training to African and other manufacturers from the developing world. www. metanoia. co. za

International Conference on Harmonization v The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) offers training programs around the world. q. ICH’s guidelines have become recognised industry standards, and have been adopted by regulatory authorities in countries such as Botswana and South Africa. q. Examples of the ICH guidelines include GMP, Stability, Qualification and Validation, Pharmaceutical Quality System, Development and Manufacture of Drug Substance among many. v ICH has conducted various training programmes for African regulators; and the SADC Regulator’s forum is represented in ICH and the organisation has invited SADC regulators to sit on various its committees. www. metanoia. co. za

World Health Organisation v The WHO provides technical assistance and training across various aspects of medicine regulation. q. One of their key programmes is WHO prequalification program, which entails working with NMRAs from developing countries q. Offers a combined WHO PQ training program for industry and regulators that is offered in conjunction with USP. q. Training in pharmaco-vigilance q. Development and validation of analytical test methods for the quality screening of medicines. q. Quality control & assurance of medicines. q. Training of regulatory officials & quality control laboratory personnel. q. Provision of support to member states in the establishment & management of quality control laboratories. www. metanoia. co. za

International Pharmaceutical Federation v The International Pharmaceutical Federation (FIP) - global federation of national associations of pharmacists and pharmaceutical scientists. qprovides various training services to its members around the globe. v The FIP’s pharmaceutical sciences group’s key focus is advancing pharmaceutical sciences through creation of global networks for sharing knowledge and information between pharmaceutical scientists. v This group also conducts regulatory educational workshops in developing countries. www. metanoia. co. za

Drug Information Association v The Drug Information Association (DIA) is a non-profit association that provides professionals in the pharmaceutical, biotechnology, medical device, and related industries a forum for facilitating knowledge exchange and collaboration through among others: q Education and training q Meetings, conferences, webinars, online learning, and other events to foster knowledge exchange, collaboration, and networking q Publications and knowledge resources v DIA, International Federation of Pharmaceutical Manufacturers Association (IFPMA), African Regulatory Network and Gates Foundation jointly host an African Regulatory Conference. q aims to encourage greater harmonisation of regulatory requirements on the African continent and covers a number of topics including; African Medicines Registration Harmonisation (AMRH), management of variations, inspections/GMP/Quality, counterfeits/pharmacovigilance/safety, transparency/good regulatory practices, dossier evaluation (requirements, frills, samples etc. ) and clinical trials www. metanoia. co. za
Dr Skhumbuzo Ngozwana MBCh. B (UCT). MPharm Med (UP) MBA (GIBS) MD – Metanoia Pharma Consulting Skhumbuzo@metanoia. co. za www. metanoia. co. za +27 82 829 3832
- Slides: 49