Bridge Course PHYSICS Bridge course syllabus 2020 2021
Bridge Course PHYSICS

Bridge course syllabus (2020 -2021) Heat Conduction-Convection-Radiation- Thermal conductivity. Newton’s law of cooling. Quantum Physics Energy Spectrum-uncertainty principle-Dual nature Physics of Sound Pitch / frequency-Loudness / Intensity-Measurement of Intensity level-decibel-Weber-Fechner law-Quality or timbre Optics Laser- Basic concepts- Communication using high energetic photons. Crystallography Unit Cell- Space Lattice- Bravais lattices- Various crystal Structure

Heat Transfer • Heat always moves from a warmer place to a cooler place. • Hot objects in a cooler room will cool to room temperature. • Cold objects in a warmer room will heat up to room temperature.
Heat Transfer Methods CONDUCTION CONVECTION RADIATION

Conduction When we heat a metal strip at one end, the heat travels to the other end. As we heat the metal, the particles vibrate, these vibrations make the adjacent particles vibrate, and so on a, thus the transmission of heat takes place by molecular vibrations in the case of conduction. As it always requires material medium(solid) it takes place in vacuum.
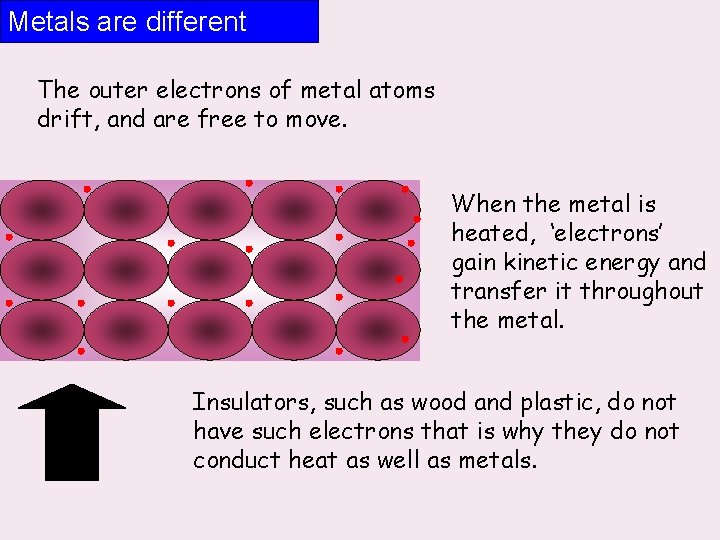
Metals are different The outer electrons of metal atoms drift, and are free to move. When the metal is heated, ‘electrons’ gain kinetic energy and transfer it throughout the metal. Insulators, such as wood and plastic, do not have such electrons that is why they do not conduct heat as well as metals.
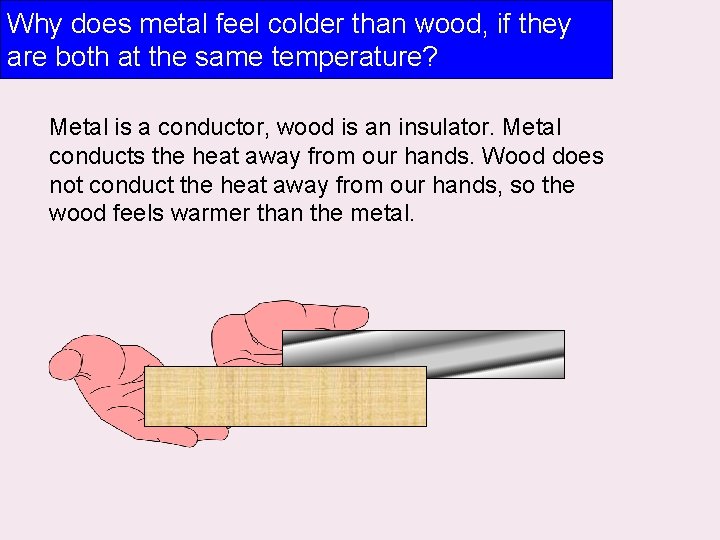
Why does metal feel colder than wood, if they are both at the same temperature? Metal is a conductor, wood is an insulator. Metal conducts the heat away from our hands. Wood does not conduct the heat away from our hands, so the wood feels warmer than the metal.
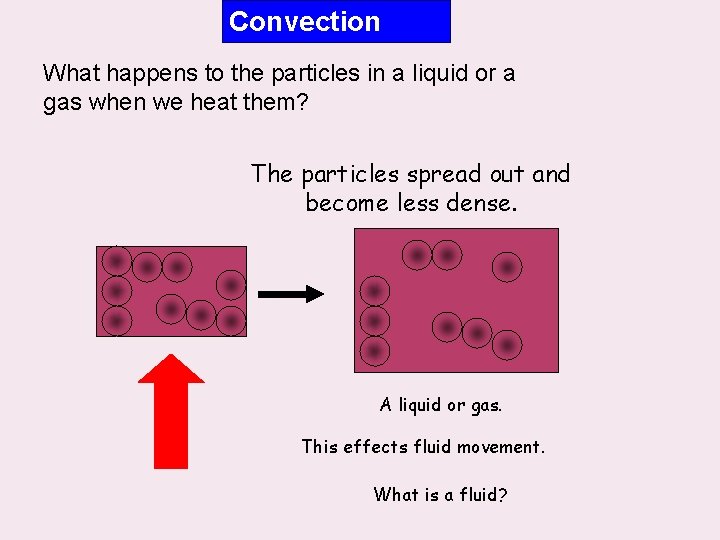
Convection What happens to the particles in a liquid or a gas when we heat them? The particles spread out and become less dense. A liquid or gas. This effects fluid movement. What is a fluid?
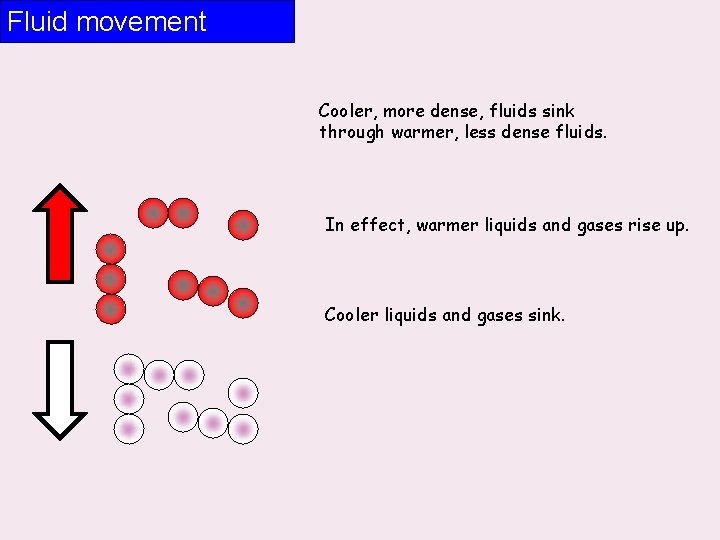
Fluid movement Cooler, more dense, fluids sink through warmer, less dense fluids. In effect, warmer liquids and gases rise up. Cooler liquids and gases sink.
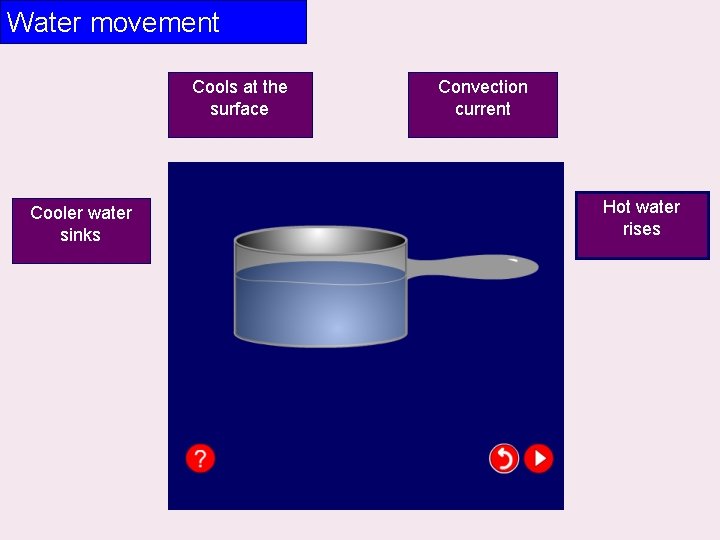
Water movement Cools at the surface Cooler water sinks Convection current Hot water rises
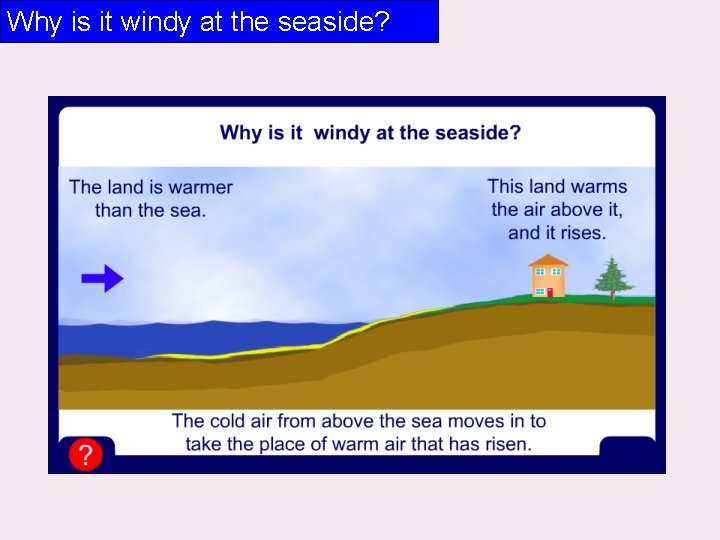
Why is it windy at the seaside?
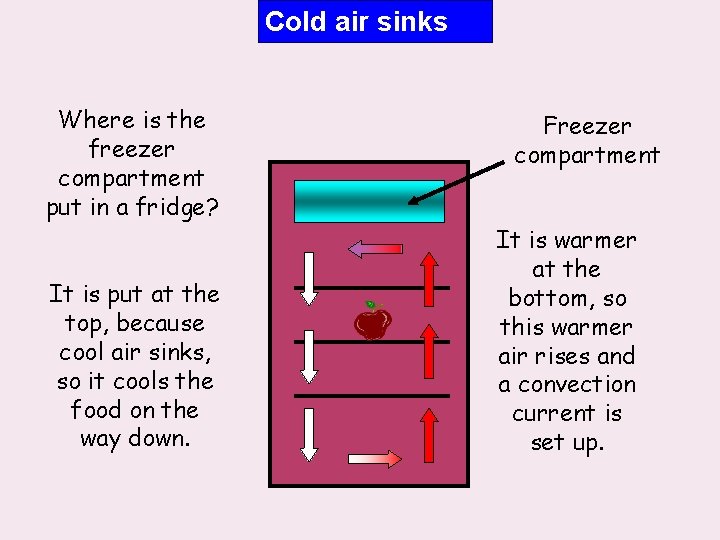
Cold air sinks Where is the freezer compartment put in a fridge? It is put at the top, because cool air sinks, so it cools the food on the way down. Freezer compartment It is warmer at the bottom, so this warmer air rises and a convection current is set up.
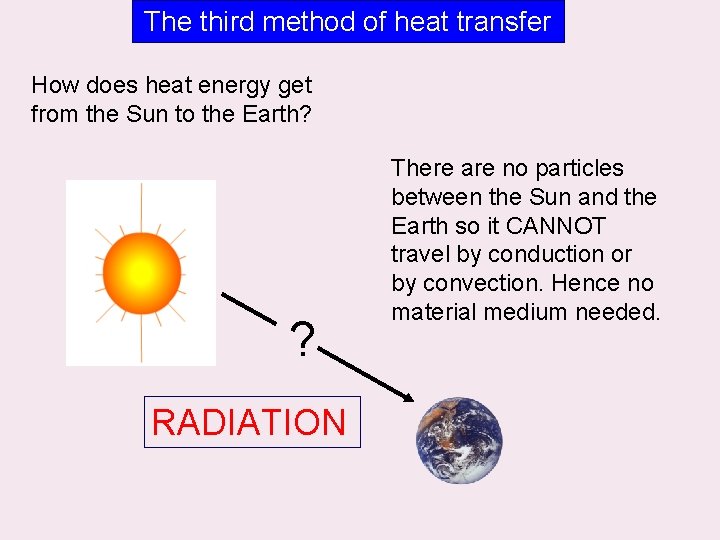
The third method of heat transfer How does heat energy get from the Sun to the Earth? ? RADIATION There are no particles between the Sun and the Earth so it CANNOT travel by conduction or by convection. Hence no material medium needed.

Convection questions Why does hot air rise and cold air sink? Cool air is more dense than warm air, so the cool air ‘falls through’ the warm air. Why are boilers placed beneath hot water tanks in people’s homes? Hot water rises. So when the boiler heats the water, and the hot water rises, the water tank is filled with hot water.

Radiation questions Why are houses painted white in hot countries? White reflects heat radiation and keeps the house cooler. Why are shiny foil blankets wrapped around marathon runners at the end of a race? The shiny metal reflects the heat radiation from the runner back in, this stops the runner getting cold.
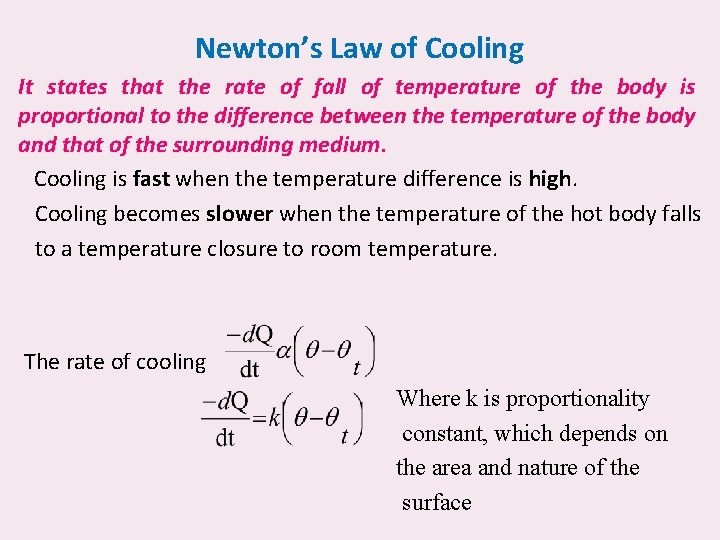
Newton’s Law of Cooling It states that the rate of fall of temperature of the body is proportional to the difference between the temperature of the body and that of the surrounding medium. Cooling is fast when the temperature difference is high. Cooling becomes slower when the temperature of the hot body falls to a temperature closure to room temperature. The rate of cooling Where k is proportionality constant, which depends on the area and nature of the surface
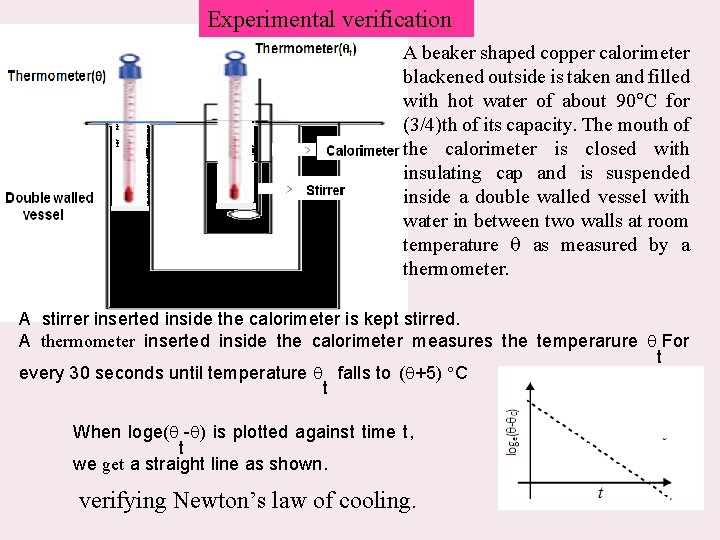
Experimental verification A beaker shaped copper calorimeter blackened outside is taken and filled with hot water of about 90 C for (3/4)th of its capacity. The mouth of the calorimeter is closed with insulating cap and is suspended inside a double walled vessel with water in between two walls at room temperature as measured by a thermometer. A stirrer inserted inside the calorimeter is kept stirred. A thermometer inserted inside the calorimeter measures the temperarure For t every 30 seconds until temperature falls to ( +5) C t When loge( - ) is plotted against time t, t we get a straight line as shown. verifying Newton’s law of cooling.
Applications of Newton’s Law of Cooling 1. Helps to design radiators/cooling system in thermal machine. 2. To calculate the time taken for a hot object to cool down to a lower temperature. 3. To determine the specific heat capacity of a substance. 4. It helps to estimate the time of death by measuring the temperature of dead body.

ORIGIN OF QUANTUM PHYSICS
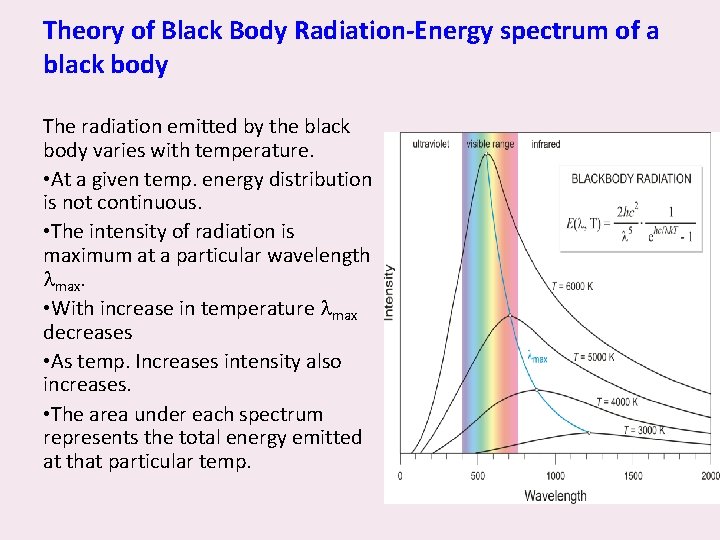
Theory of Black Body Radiation-Energy spectrum of a black body The radiation emitted by the black body varies with temperature. • At a given temp. energy distribution is not continuous. • The intensity of radiation is maximum at a particular wavelength max. • With increase in temperature max decreases • As temp. Increases intensity also increases. • The area under each spectrum represents the total energy emitted at that particular temp.
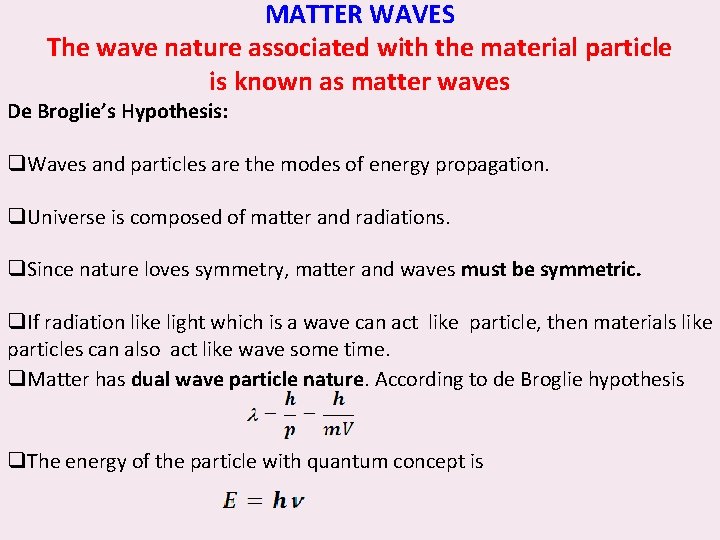
MATTER WAVES The wave nature associated with the material particle is known as matter waves De Broglie’s Hypothesis: q. Waves and particles are the modes of energy propagation. q. Universe is composed of matter and radiations. q. Since nature loves symmetry, matter and waves must be symmetric. q. If radiation like light which is a wave can act like particle, then materials like particles can also act like wave some time. q. Matter has dual wave particle nature. According to de Broglie hypothesis q. The energy of the particle with quantum concept is
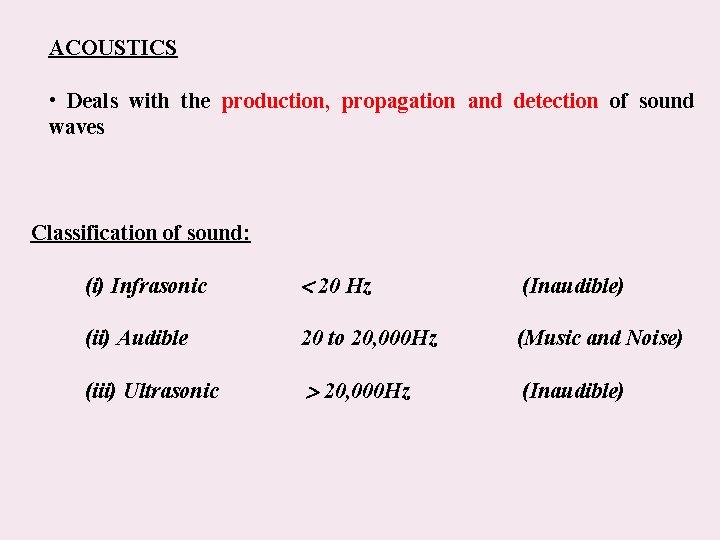
ACOUSTICS • Deals with the production, propagation and detection of sound waves Classification of sound: (i) Infrasonic 20 Hz (Inaudible) (ii) Audible 20 to 20, 000 Hz (Music and Noise) (iii) Ultrasonic 20, 000 Hz (Inaudible)

Characteristics of Musical sound: (i) Pitch or frequency Pitch : - a degree of sensation depends on frequency Frequency: number of vibrations of sound producing object/second High frequency – shrill sound- voice of ladies, children, mosquito Low frequency - grave sound- sound by lion
ii) Quality or Timbre • Distinguish b/w any two or more musical sound having same pitch and frequency • Smallest frequency is called fundamental and frequencies accompanying fundamental are called overtones. (iii) Intensity or Loudness Intensity : amount of sound energy flowing per sec per unit area I = Q /A watt/m 2 Loudness : degree of sensation varies from one observer from other
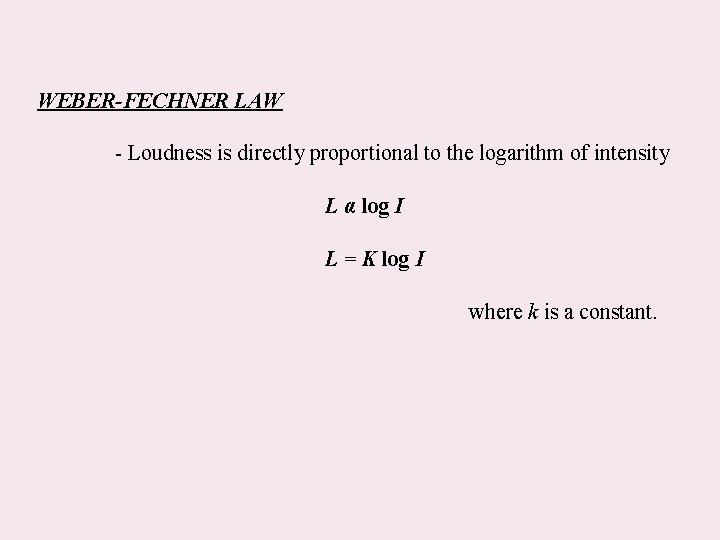
WEBER-FECHNER LAW - Loudness is directly proportional to the logarithm of intensity L α log I L = K log I where k is a constant.
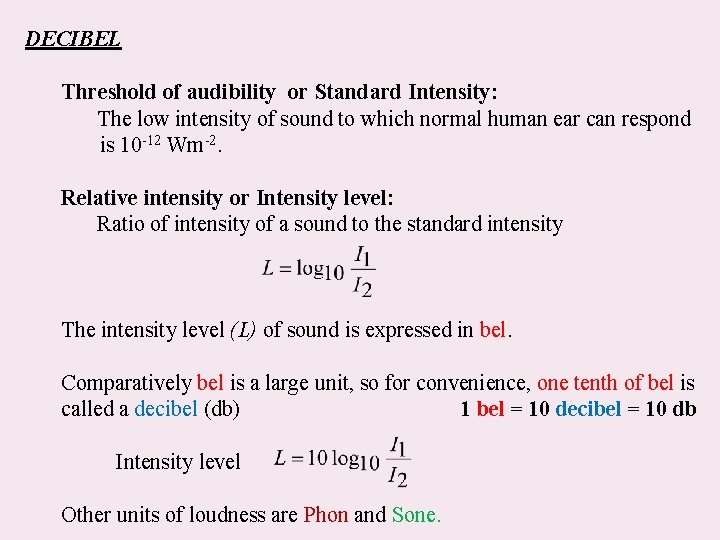
DECIBEL Threshold of audibility or Standard Intensity: The low intensity of sound to which normal human ear can respond is 10 -12 Wm-2. Relative intensity or Intensity level: Ratio of intensity of a sound to the standard intensity The intensity level (L) of sound is expressed in bel. Comparatively bel is a large unit, so for convenience, one tenth of bel is called a decibel (db) 1 bel = 10 decibel = 10 db Intensity level Other units of loudness are Phon and Sone.
Basics of laser ˝LASER˝ is acronym of expansion Light Amplification by Stimulated Emission of Radiation If the light passes through the medium, it experiences reflection, refraction and scattering losses. Then, How did the amplification of light appears as laser ?

Conditions for laser action The medium on which the light propagates should be altered for the following : 1. There must be population inversion (N 2>N 1) in the atoms of medium 2. The energy density of radiation on the medium should be increased. The ρ(v) is to be increased in optical resonance cavity. 3. The ratio of Einstein’s coefficient should be less (A 21/B 21<) so that more stimulated emission to take place than the spontaneous emission
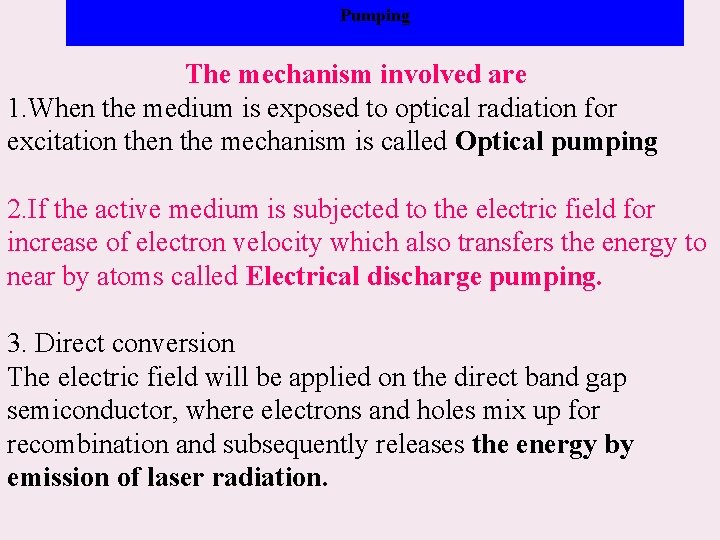
Pumping The mechanism involved are: 1. When the medium is exposed to optical radiation for excitation the mechanism is called Optical pumping 2. If the active medium is subjected to the electric field for increase of electron velocity which also transfers the energy to near by atoms called Electrical discharge pumping. 3. Direct conversion The electric field will be applied on the direct band gap semiconductor, where electrons and holes mix up for recombination and subsequently releases the energy by emission of laser radiation.

Pumping 4. Pumping by inelastic collision In an inelastic collision of atoms with electron, one of atom will be excited. The atom simultaneously releases the energy gained in the collision to its neighbor and make it become excited known as pumping by inelastic collision
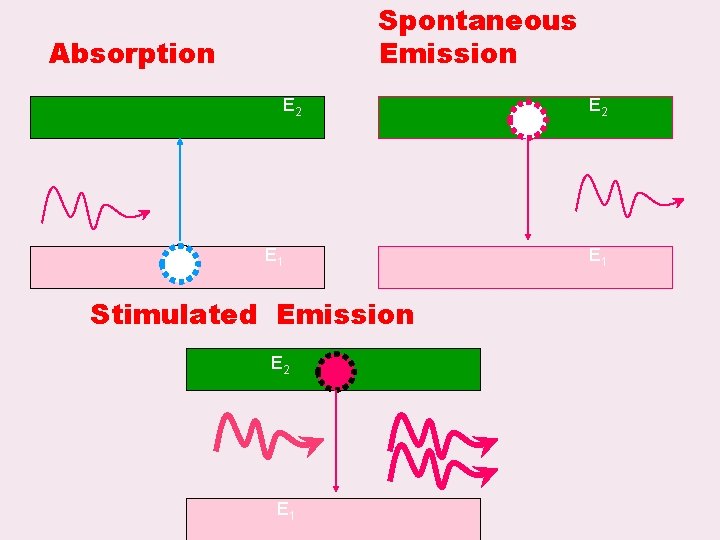
Spontaneous Emission Absorption E 2 E 1 Stimulated Emission E 2 E 1

Optical fibre • A thin flexible and transparent wire prepared for light propagation • The optical fibre has been constructed for the following reasons: 1. The light wave cannot traverse long distance in air without any losses –air friction and other related losses. 2. To make loss less light wave propagation, the optical waves can be guided through optical fibre.
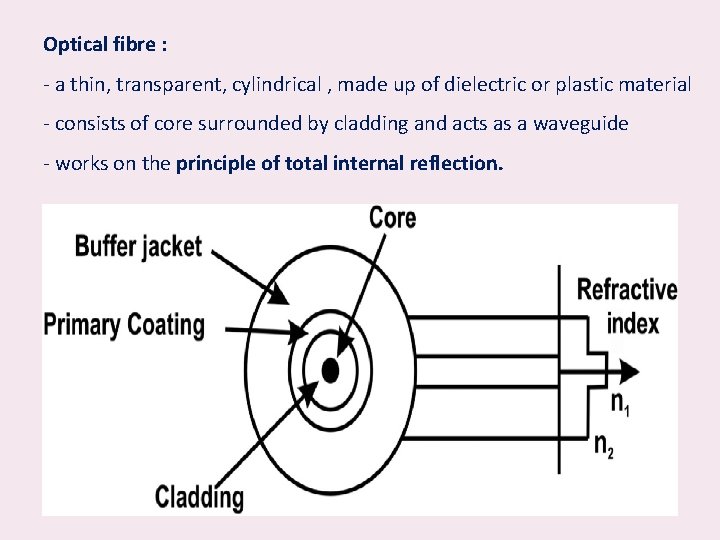
Optical fibre : - a thin, transparent, cylindrical , made up of dielectric or plastic material - consists of core surrounded by cladding and acts as a waveguide - works on the principle of total internal reflection.
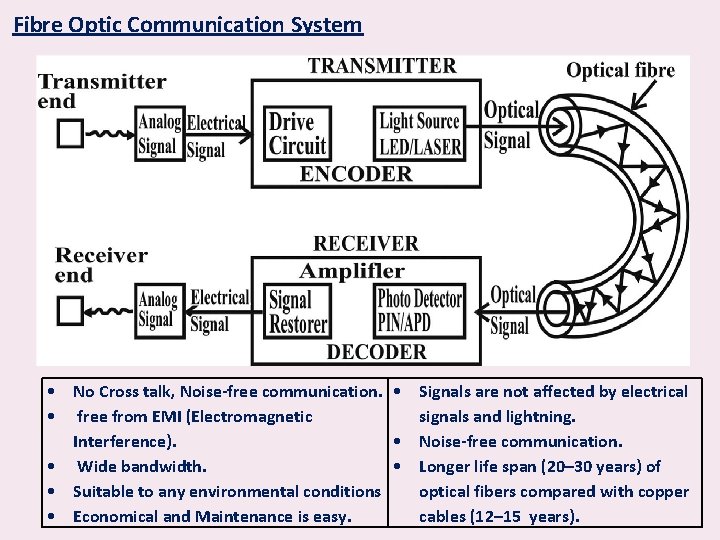
Fibre Optic Communication System • No Cross talk, Noise-free communication. • Signals are not affected by electrical • free from EMI (Electromagnetic signals and lightning. Interference). • Noise-free communication. • Wide bandwidth. • Longer life span (20– 30 years) of • Suitable to any environmental conditions optical fibers compared with copper • Economical and Maintenance is easy. cables (12– 15 years).

INTRODUCTION TO CRYSTAL PHYSICS ‘What is Crystal Physics? Crystal Physics’ or ‘Crystallography’ is a branch of physics that deals with the study of all possible types of crystals and the physical properties of crystalline solids by the determination of their actual structure by using X-rays, neutron beams and electron beams. 35
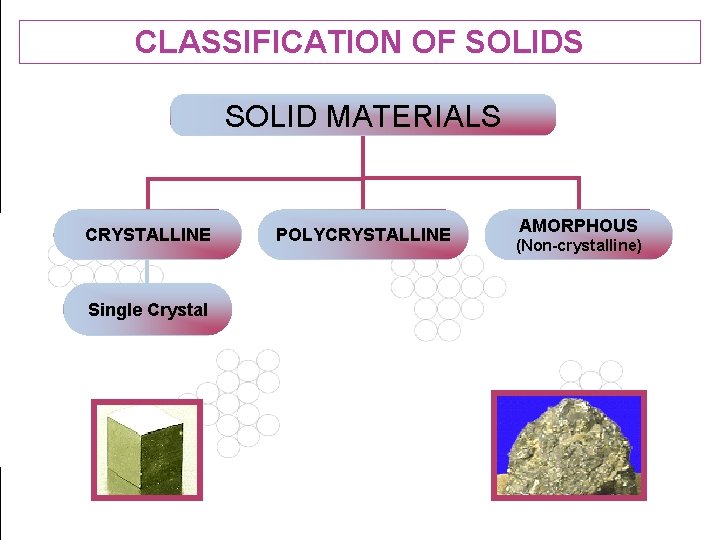
CLASSIFICATION OF SOLIDS SOLID MATERIALS CRYSTALLINE POLYCRYSTALLINE AMORPHOUS (Non-crystalline) Single Crystal 36
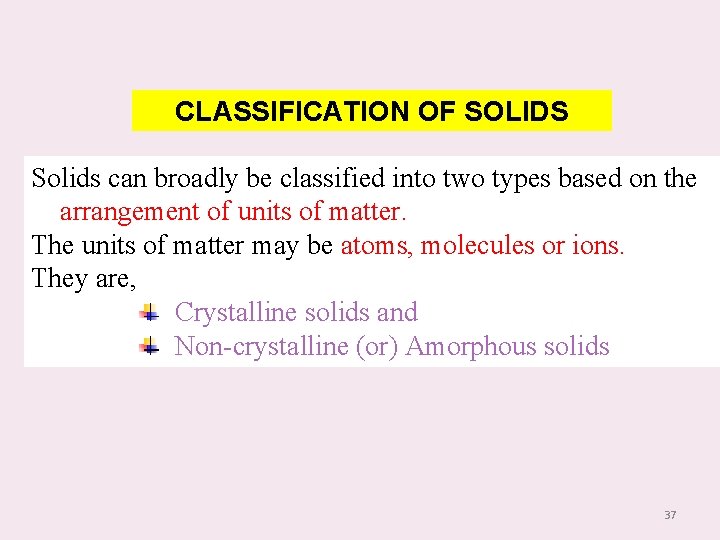
CLASSIFICATION OF SOLIDS Solids can broadly be classified into two types based on the arrangement of units of matter. The units of matter may be atoms, molecules or ions. They are, Crystalline solids and Non-crystalline (or) Amorphous solids 37

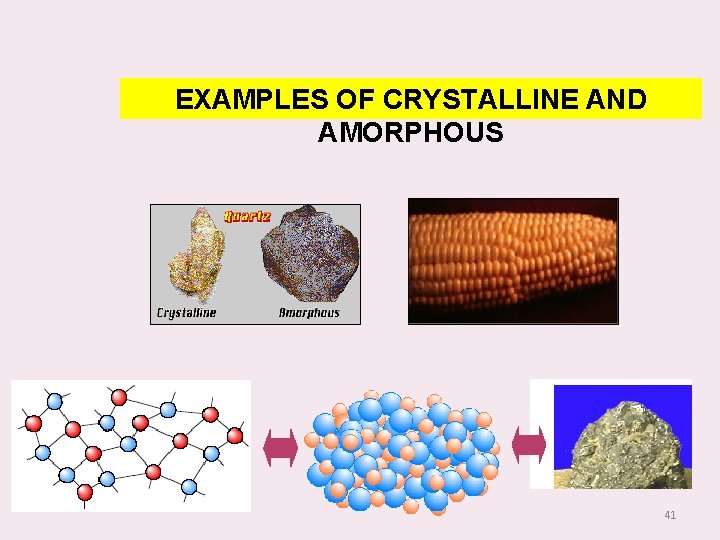
CRYSTALLINE SOLIDS A substance is said to be crystalline when the arrangement of units of matter is regular and periodic. A crystalline material has directional properties and therefore called as anisotropic substance. A crystal has a sharp melting point. It possesses a regular shape and if it is broken, all broken pieces have the same regular shape. - 38

CRYSTALLINE SOLIDS A crystalline material can either be a single (mono) crystal or a polycrystal. A single crystal consists of only one crystal, whereas the polycrystalline material consists of many crystals separated by well-defined boundaries. Examples Metallic crystals – Cu, Ag, Al, Mg etc, Non-metallic crystals – Carbon, Silicon, Germanium 39

NON CRYSTALLINE SOLIDS In amorphous solids, the constituent particles arranged in an orderly manner. They are randomly distributed. They do not have directional properties and so they are called as `isotropic’ substances. They have wide range of melting point and do not possess a regular shape. Examples: Glass, Plastics, Rubber etc. , 40
EXAMPLES OF CRYSTALLINE AND AMORPHOUS 41
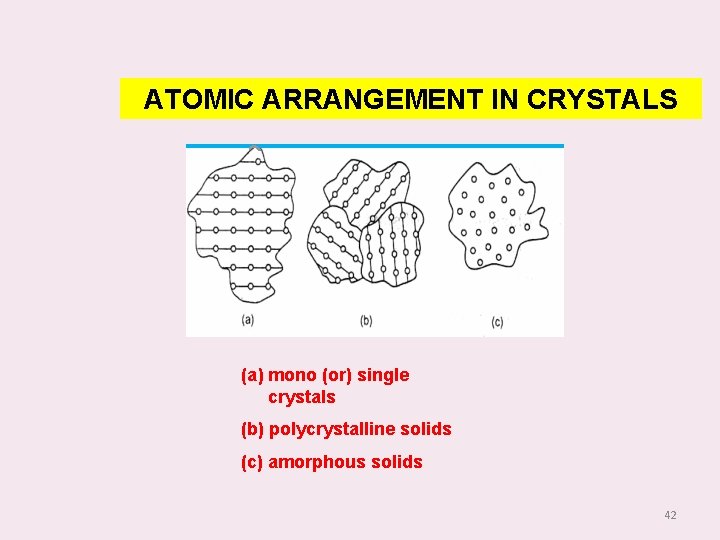
ATOMIC ARRANGEMENT IN CRYSTALS (a) mono (or) single crystals (b) polycrystalline solids (c) amorphous solids 42
SPACE LATTICE A lattice is a regular and periodic arrangement of points in three dimension. It is defined as an infinite array of points in three dimension in which every point has surroundings identical to that of every other point in the array. The Space lattice is otherwise called the Crystal lattice 43

BASIS A crystal structure is formed by associating every lattice point with an unit assembly of atoms or molecules identical in composition, arrangement and orientation. This unit assembly is called the `basis’. When the basis is repeated with correct periodicity in all directions, it gives the actual crystal structure. The crystal structure is real, while the lattice is imaginary. 44
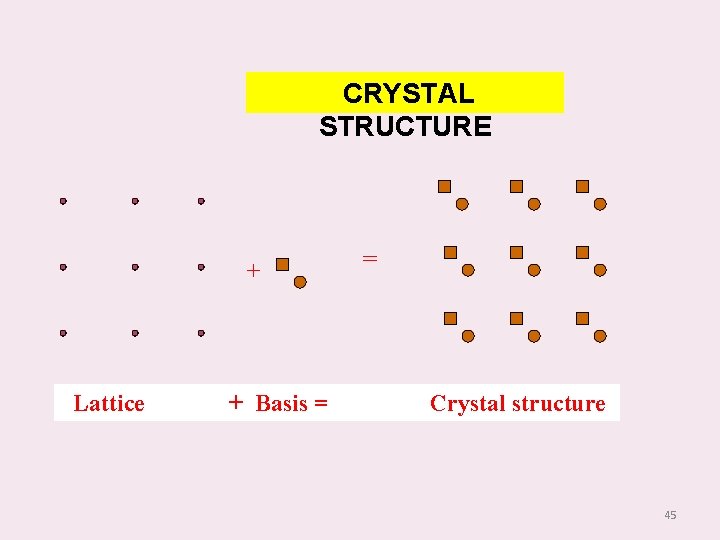
CRYSTAL STRUCTURE + Lattice + Basis = = Crystal structure 45
UNIT CELL A unit cell is defined as a fundamental building block of a crystal structure, which can generate the complete crystal by repeating its own dimensions in various directions. 46
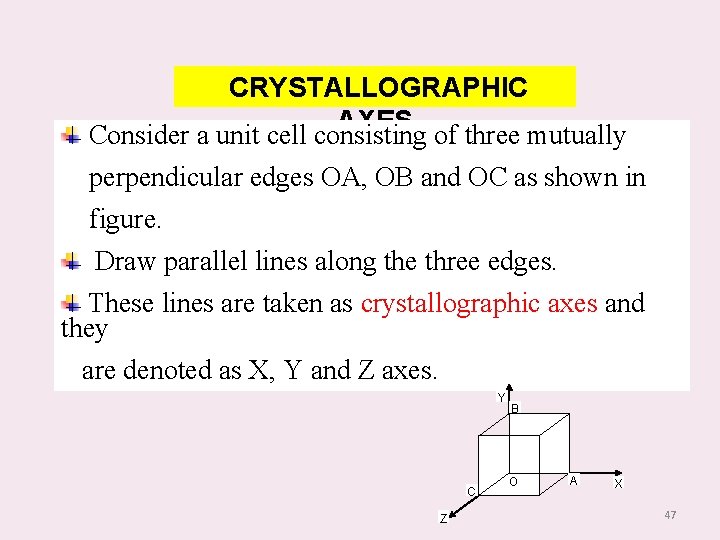
CRYSTALLOGRAPHIC AXES of three mutually Consider a unit cell consisting perpendicular edges OA, OB and OC as shown in figure. Draw parallel lines along the three edges. These lines are taken as crystallographic axes and they are denoted as X, Y and Z axes. Y C Z B O A X 47

CRYSTALS SYSTEMS The seven systems are, Cubic (isometric) Tetragonal Orthorhombic Trigonal (rhombohedral) Monoclinic Triclinic Hexagonal 48
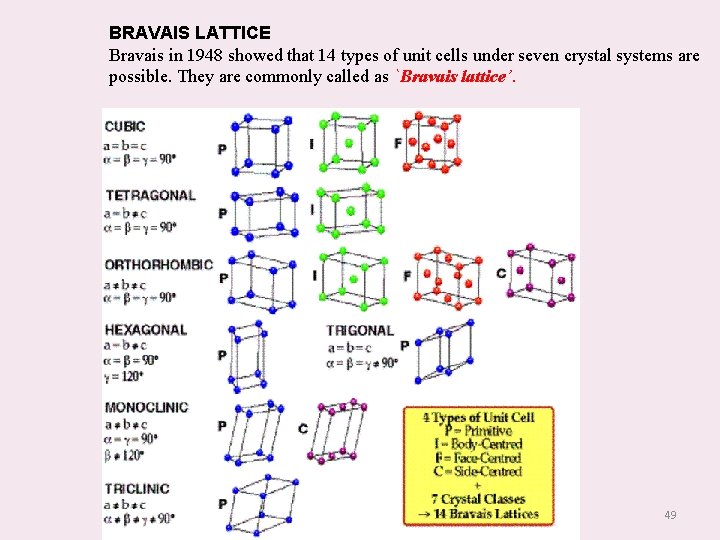
BRAVAIS LATTICE Bravais in 1948 showed that 14 types of unit cells under seven crystal systems are possible. They are commonly called as `Bravais lattice’. 49
- Slides: 49