Breakthroughs in IPF Management The Next Steps in

Breakthroughs in IPF Management: The Next Steps in Individualizing Treatment

Learning Objectives As a result of attending this activity, participants should be able to: • Select appropriate therapies that reflect current evidence and/ or widely accepted guidelines that are individualized to patients • Utilize dose modification to manage side effects in patients • Demonstrate ability to manage comorbid conditions associated with IPF

Case #1: Maria

Maria • 69 -year-old white woman • Presentation – – Progressive shortness of breath on exertion for 6 months Dry hacking cough for 3 months Complains of joint stiffness in morning No other constitutional symptoms • Habits – Former 1 PPD smoker x 15 years, stopped 10 years ago – Social Et. OH • Social – Has a cat at home – No bird or any other significant exposures • Occupation: line worker in auto assembly plant x 15 y • Presented to her PCP who obtained a chest X-ray and then referred her to a pulmonologist for further work-up
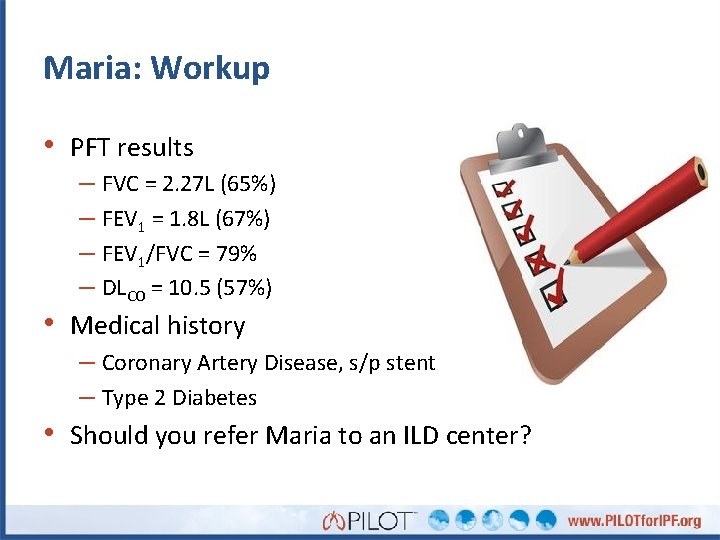
Maria: Workup • PFT results – FVC = 2. 27 L (65%) – FEV 1 = 1. 8 L (67%) – FEV 1/FVC = 79% – DLCO = 10. 5 (57%) • Medical history – Coronary Artery Disease, s/p stent – Type 2 Diabetes • Should you refer Maria to an ILD center?
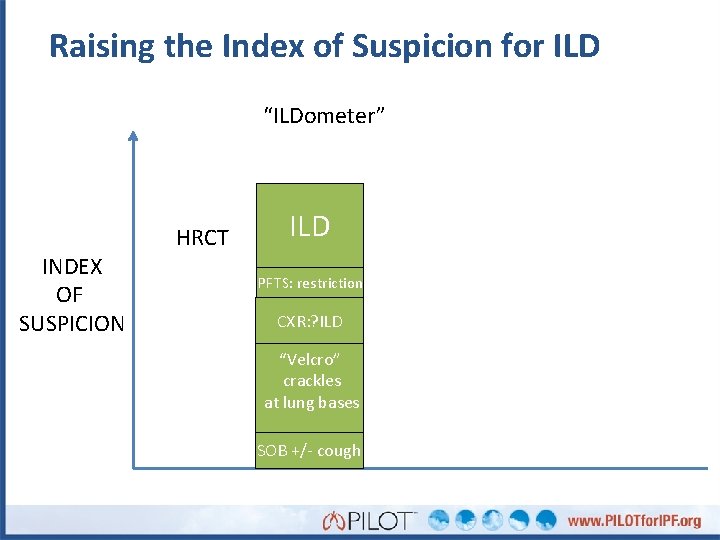
Raising the Index of Suspicion for ILD “ILDometer” INDEX OF SUSPICION HRCT ILD PFTS: restriction CXR: ? ILD “Velcro” crackles at lung bases SOB +/- cough
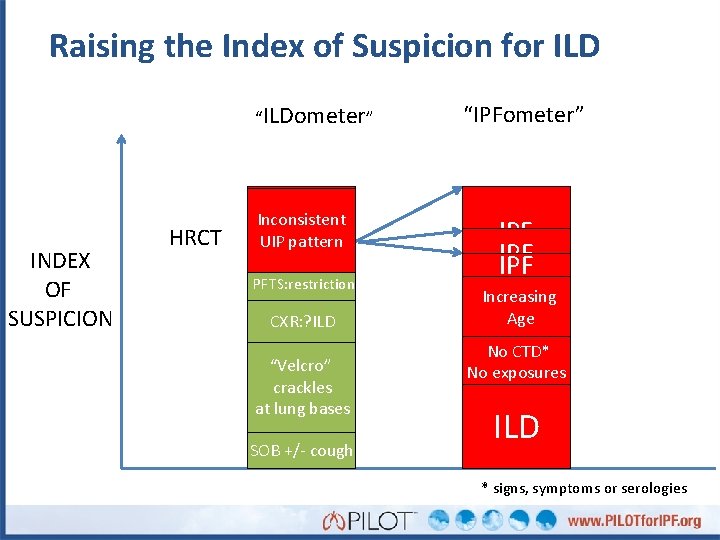
Raising the Index of Suspicion for ILD “ILDometer” INDEX OF SUSPICION HRCT UIP Possible Inconsistent UIP pattern ILD PFTS: restriction CXR: ? ILD “Velcro” crackles at lung bases SOB +/- cough “IPFometer” IPF IPF Increasing Age No CTD* No exposures ILD * signs, symptoms or serologies
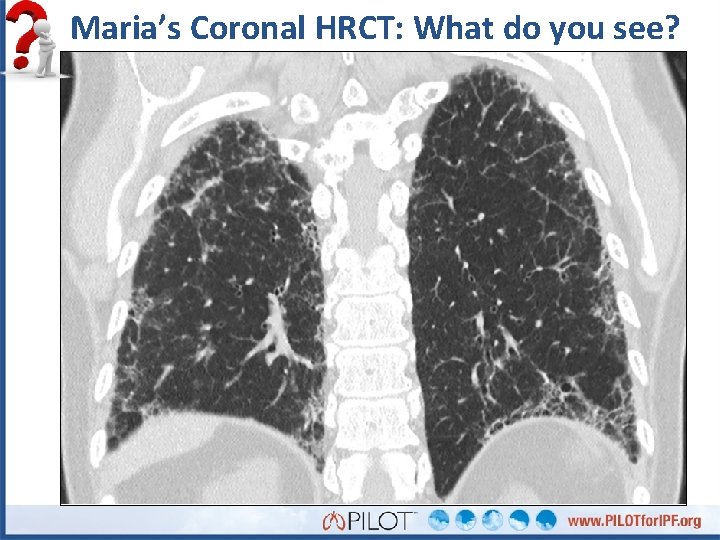
Maria’s Coronal HRCT: What do you see?
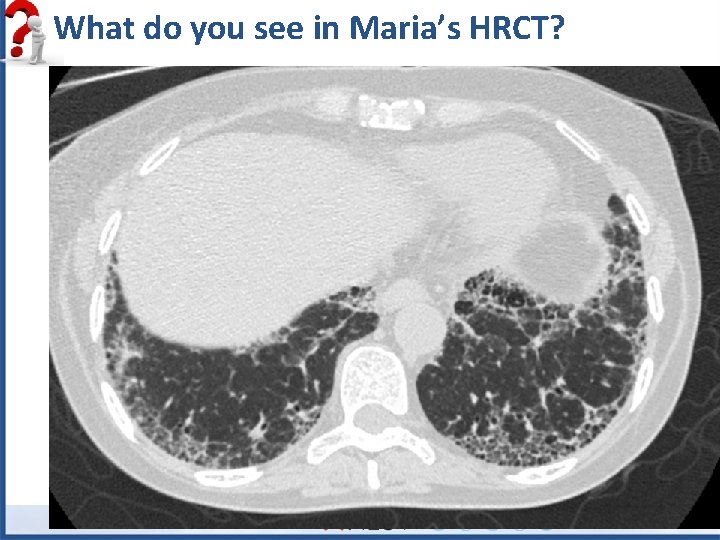
What do you see in Maria’s HRCT?
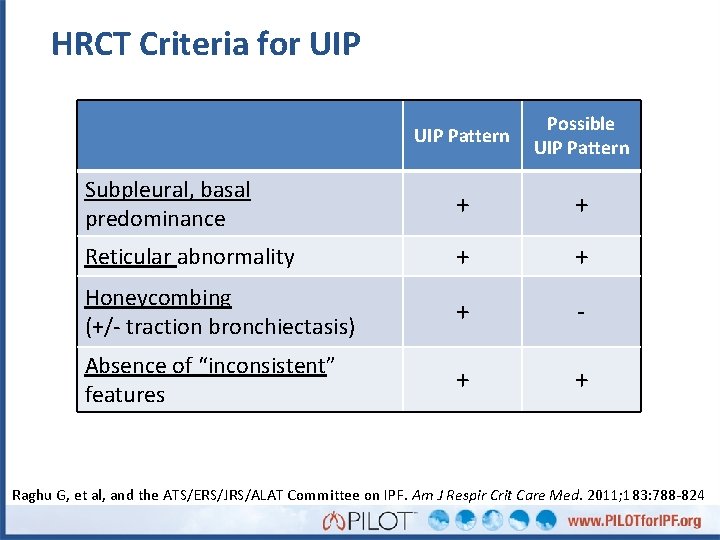
HRCT Criteria for UIP Pattern Possible UIP Pattern Subpleural, basal predominance + + Reticular abnormality + + Honeycombing (+/- traction bronchiectasis) + - Absence of “inconsistent” features + + Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
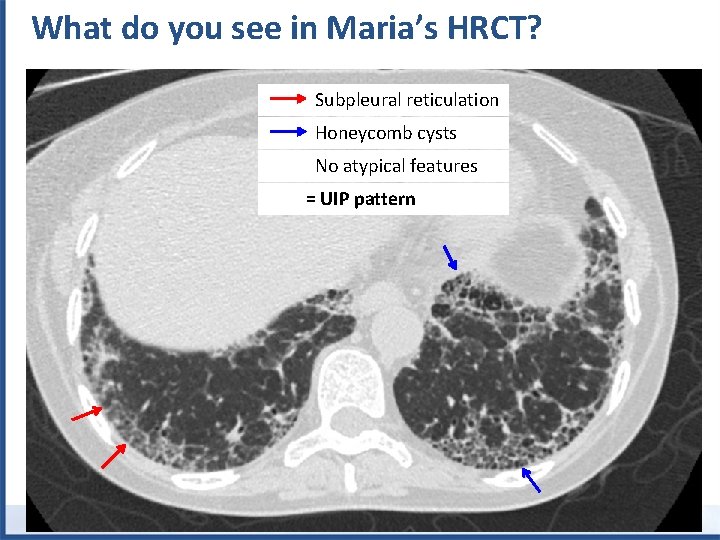
What do you see in Maria’s HRCT? Subpleural reticulation Honeycomb cysts No atypical features = UIP pattern
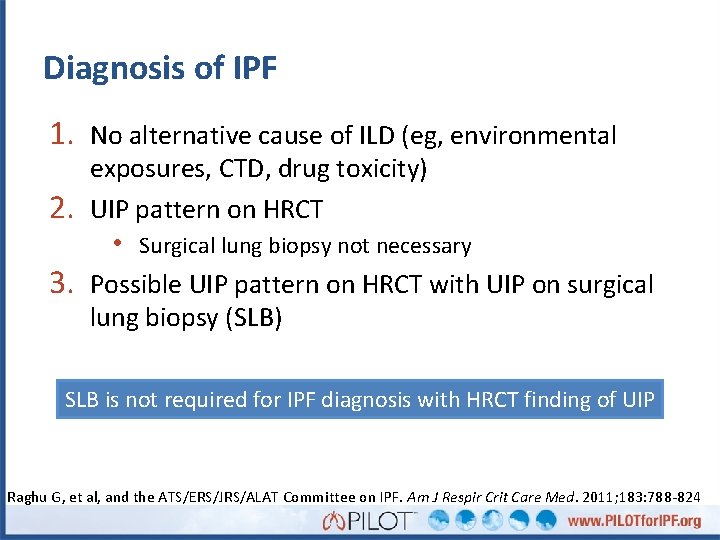
Diagnosis of IPF 1. No alternative cause of ILD (eg, environmental 2. 3. exposures, CTD, drug toxicity) UIP pattern on HRCT • Surgical lung biopsy not necessary Possible UIP pattern on HRCT with UIP on surgical lung biopsy (SLB) SLB is not required for IPF diagnosis with HRCT finding of UIP Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824

What is your next step to establish Maria’s diagnosis? A. B. C. D. E. Surgical lung biopsy Transbronchial biopsy Serologies (connective tissue disease, HP panel) Referral to rheumatology Diagnosis is clear, no more information is necessary

What is your next step to establish Maria’s diagnosis? A. B. C. D. E. Surgical lung biopsy Transbronchial biopsy Serologies Maria’s serologies were negative Referral to rheumatology Diagnosis is clear, no more information is necessary Is Maria’s diagnostic process different from what you would normally do?
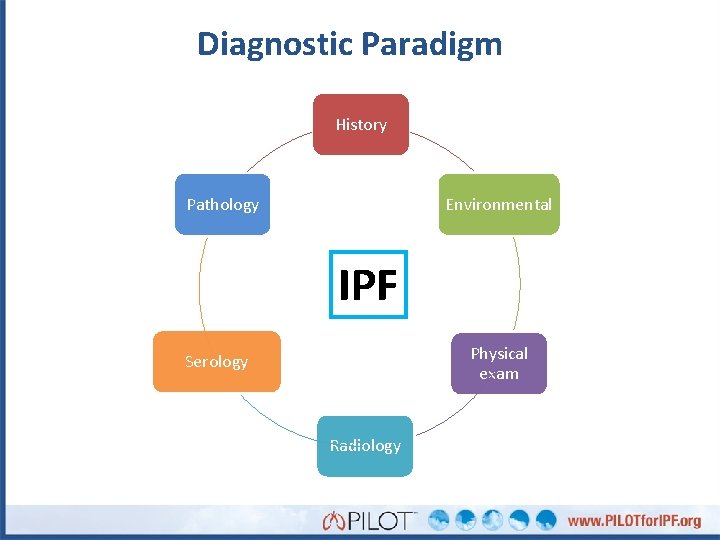
Diagnostic Paradigm History Pathology Environmental IPF Physical exam Serology Radiology
Which therapy would you recommend for Maria? Nintedanib (Ofev) Pirfenidone (Esbriet)
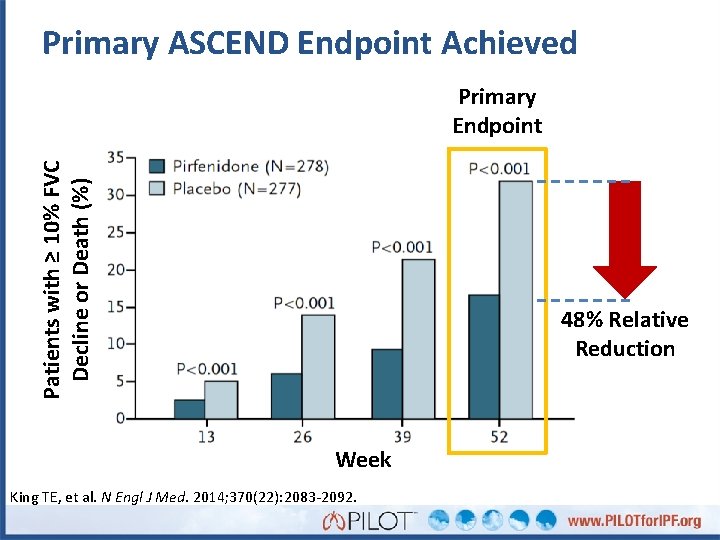
Primary ASCEND Endpoint Achieved Patients with ≥ 10% FVC Decline or Death (%) Primary Endpoint 48% Relative Reduction Week King TE, et al. N Engl J Med. 2014; 370(22): 2083 -2092.
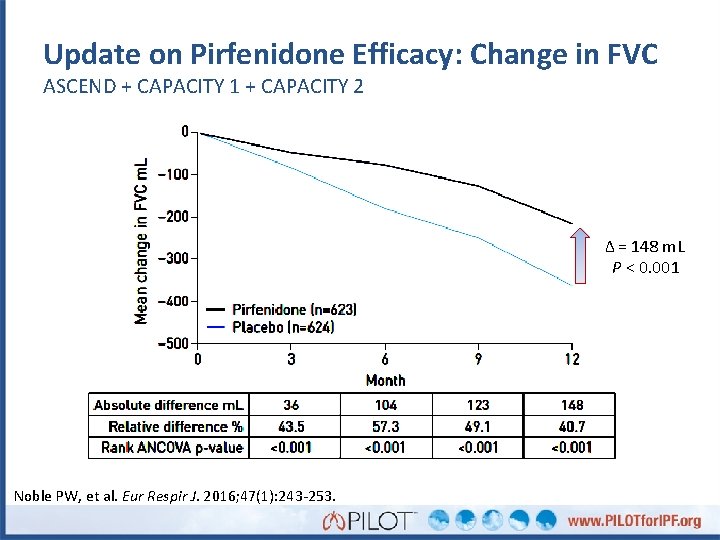
Update on Pirfenidone Efficacy: Change in FVC ASCEND + CAPACITY 1 + CAPACITY 2 Δ = 148 m. L P < 0. 001 Noble PW, et al. Eur Respir J. 2016; 47(1): 243 -253.
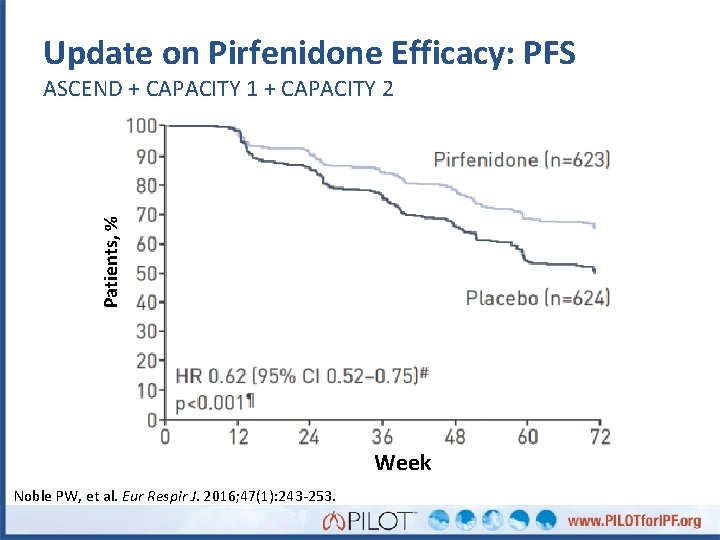
Update on Pirfenidone Efficacy: PFS Patients, % ASCEND + CAPACITY 1 + CAPACITY 2 Week Noble PW, et al. Eur Respir J. 2016; 47(1): 243 -253.
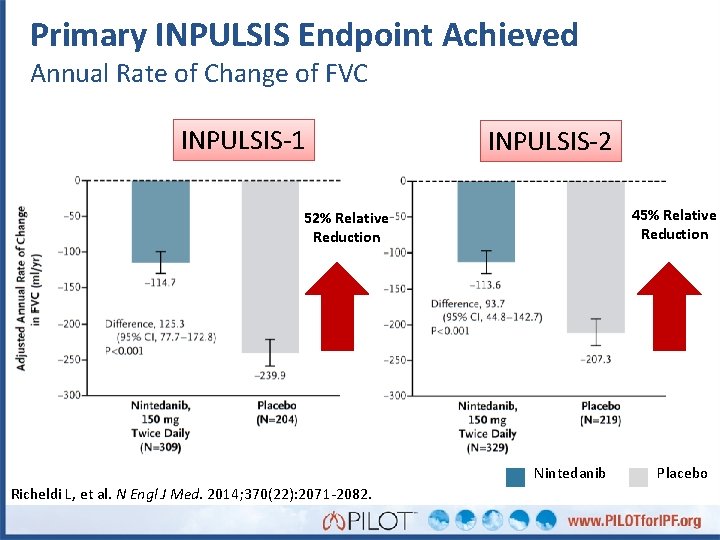
Primary INPULSIS Endpoint Achieved Annual Rate of Change of FVC INPULSIS-1 INPULSIS-2 45% Relative Reduction 52% Relative Reduction Nintedanib Richeldi L, et al. N Engl J Med. 2014; 370(22): 2071 -2082. Placebo
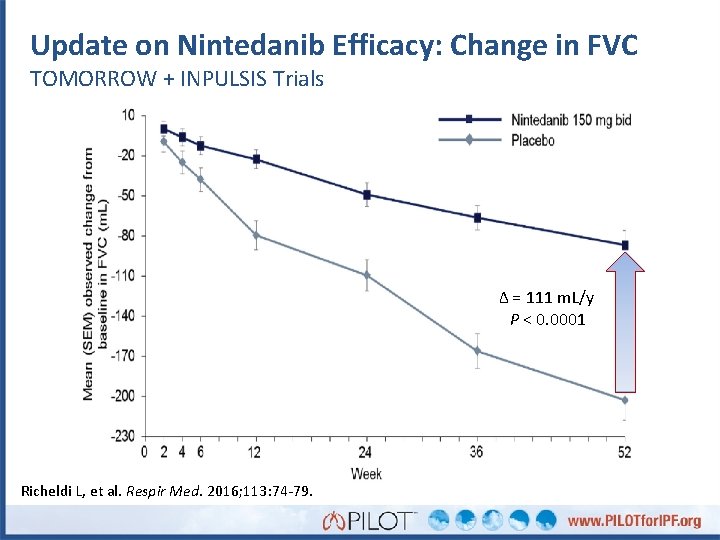
Update on Nintedanib Efficacy: Change in FVC TOMORROW + INPULSIS Trials Δ = 111 m. L/y P < 0. 0001 Richeldi L, et al. Respir Med. 2016; 113: 74 -79.
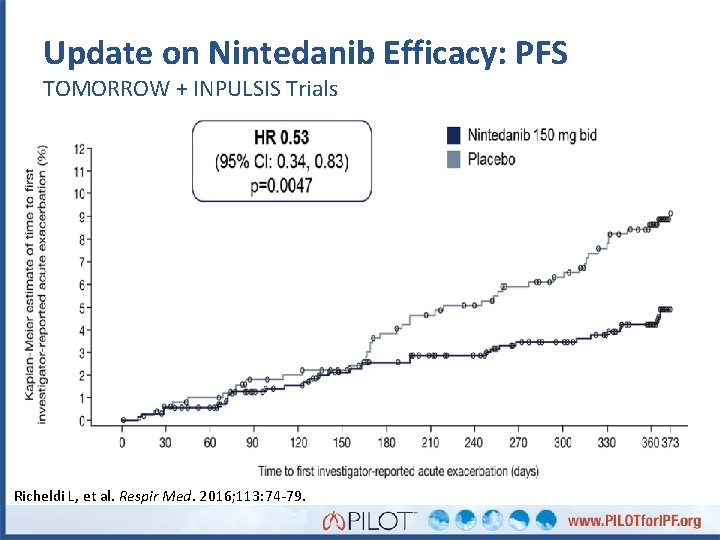
Update on Nintedanib Efficacy: PFS TOMORROW + INPULSIS Trials Richeldi L, et al. Respir Med. 2016; 113: 74 -79.
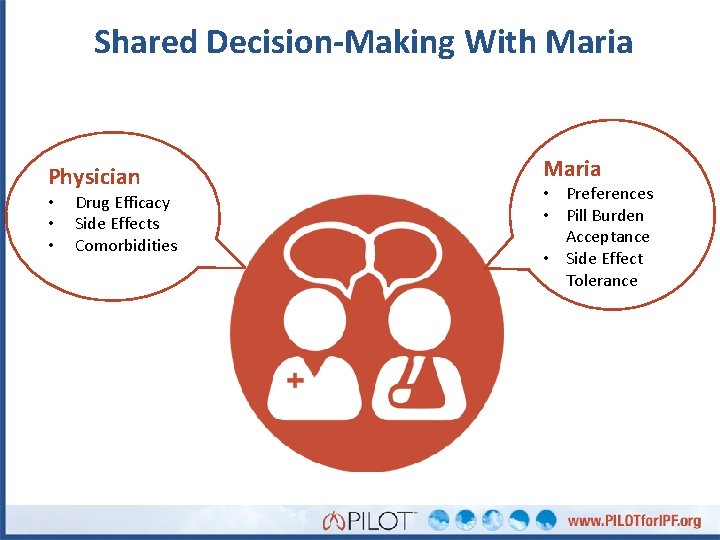
Shared Decision-Making With Maria Physician • • • Drug Efficacy Side Effects Comorbidities Maria • Preferences • Pill Burden Acceptance • Side Effect Tolerance
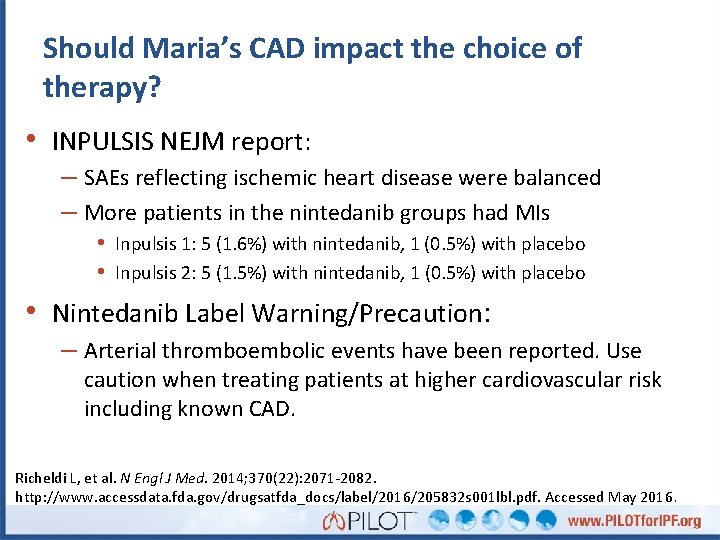
Should Maria’s CAD impact the choice of therapy? • INPULSIS NEJM report: – SAEs reflecting ischemic heart disease were balanced – More patients in the nintedanib groups had MIs • Inpulsis 1: 5 (1. 6%) with nintedanib, 1 (0. 5%) with placebo • Inpulsis 2: 5 (1. 5%) with nintedanib, 1 (0. 5%) with placebo • Nintedanib Label Warning/Precaution: – Arterial thromboembolic events have been reported. Use caution when treating patients at higher cardiovascular risk including known CAD. Richeldi L, et al. N Engl J Med. 2014; 370(22): 2071 -2082. http: //www. accessdata. fda. gov/drugsatfda_docs/label/2016/205832 s 001 lbl. pdf. Accessed May 2016.
Next Steps for Maria • Would you recommend an IPF drug for Maria? • Which one would you choose? • Which factors were important for your recommendation?

Well, here’s what happened…… • Physician and patient agreed on pirfenidone – Maria was instructed to take her medication with food • Liver enzymes monitored monthly: normal • She experienced some indigestion with pirfenidone, especially when taken on empty stomach

What is the best way to address Maria’s GI symptoms? A. B. C. D. E. Hold pirfenidone Dose reduction protocol Switch to nintedanib Switch to prednisone/NAC/azathioprine
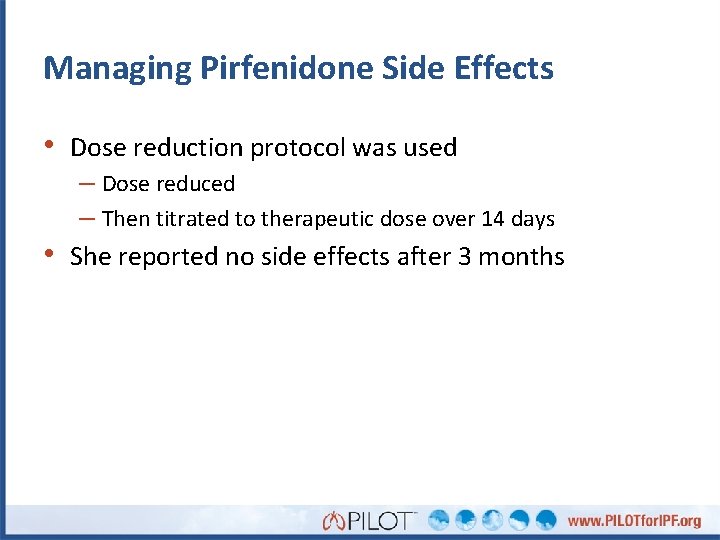
Managing Pirfenidone Side Effects • Dose reduction protocol was used – Dose reduced – Then titrated to therapeutic dose over 14 days • She reported no side effects after 3 months

What would you put on an IPF patient management checklist? • • •

IPF Management q Risk factor reduction q Discussion about nintedanib and q Patient education pirfenidone – Advocacy group q Pulmonary involvement q Focus on comorbidities – GERD, OSA, CAD, PH, VTE etc. rehabilitation q Clinical trials q Lung transplant evaluation q Supplemental oxygen q Mental health needs q Address end of life q Age-appropriate vaccinations issues: Palliative and hospice care
Maria Progresses • After 1 year PFTs decline: – FVC = 52% (> 10% decline) – FEV 1 = 47% • HRCT shows progression • You refer Maria for lung transplantation evaluation • How would you manage Maria’s medication? A. B. C. D. Continue pirfenidone Increase pirfenidone dose Switch to nintedanib Add NAC to pirfenidone
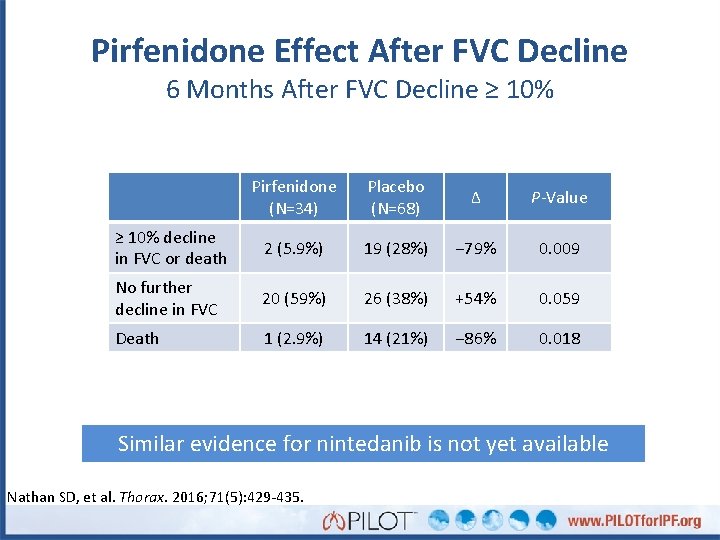
Pirfenidone Effect After FVC Decline 6 Months After FVC Decline ≥ 10% Pirfenidone (N=34) Placebo (N=68) Δ P-Value ≥ 10% decline in FVC or death 2 (5. 9%) 19 (28%) − 79% 0. 009 No further decline in FVC 20 (59%) 26 (38%) +54% 0. 059 Death 1 (2. 9%) 14 (21%) − 86% 0. 018 Similar evidence for nintedanib is not yet available Nathan SD, et al. Thorax. 2016; 71(5): 429 -435.
Maria is concerned about an acute exacerbation • Acute respiratory decline • ~5– 10% of patients/year • Criteria – Unexplained worsening of dyspnea within 1 month – Evidence of hypoxemia as defined by worsened or severely impaired gas exchange – New radiographic alveolar infiltrates – Absence of an alternative explanation such as • Infection • Pulmonary embolism • Pneumothorax or • Heart failure Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
Maria Summary • Clinical and serologic features plus definite UIP on • • • HRCT enabled definitive diagnosis without histology CAD comorbidity and patient preference led to choice of pirfenidone Side effects managed with dose reduction and titration IPF progressed despite therapy – Therapy was continued despite progression

Case #2: Henry

Presentation • 66 -year-old male • Pre-op chest X-ray for a hernia repair 6 years prior showed • • “mild lung fibrosis” Over the subsequent years, he had episodic shortness of breath-treated for “recurrent pneumonias” 1 year ago, his exertional dyspnea increased – Progressed to SOB after walking up one flight of stairs 3 months ago, consulted with primary care physician – CXR obtained which showed worsening fibrosis HRCT ordered and referred to you for diagnosis and therapy

History • PMHx – Type 2 DM – GERD • No relevant exposures • Hobbies: avid golfer • Current medications – Metformin – ASA – Fish oil – MVI
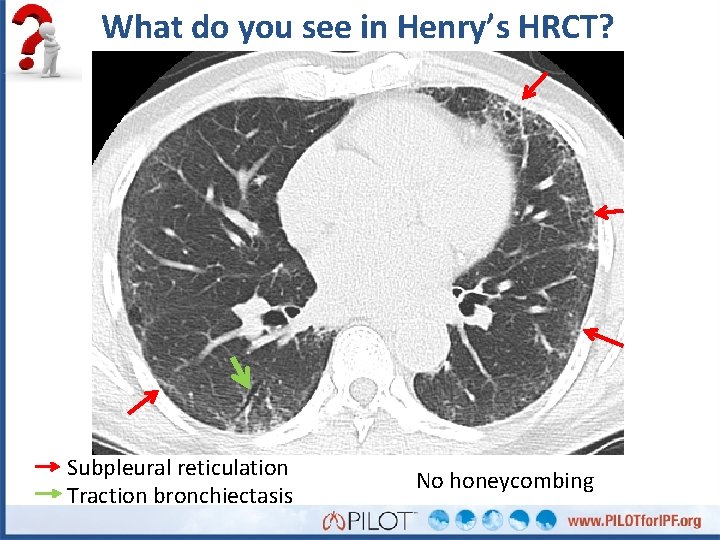
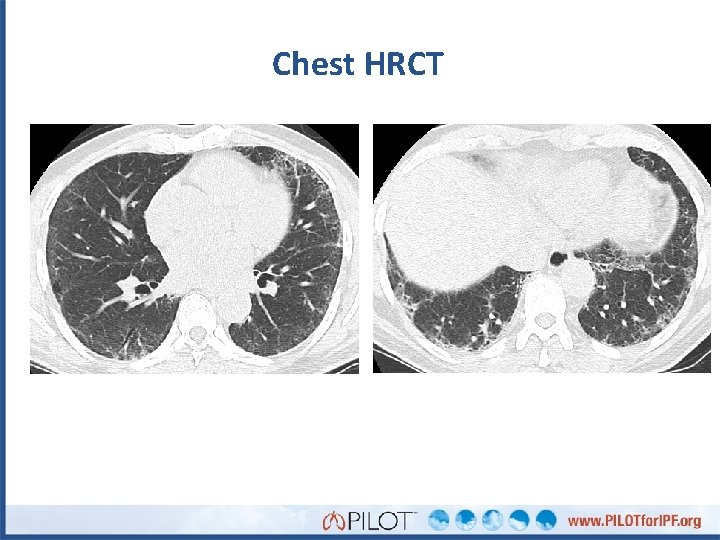
What do you see in Henry’s HRCT? Subpleural reticulation Traction bronchiectasis No honeycombing
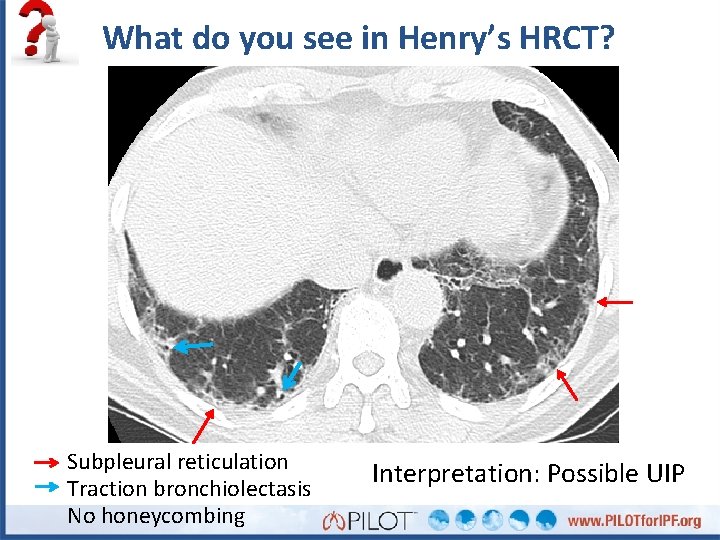
What do you see in Henry’s HRCT? Subpleural reticulation Traction bronchiolectasis No honeycombing Interpretation: Possible UIP

What is your next step to establish Henry’s diagnosis? A. B. C. D. E. Surgical lung biopsy Transbronchial biopsy Serologies (connective tissue disease, HP panel) Referral to rheumatology Diagnosis is clear, no more information is necessary
What is your next step to establish Henry’s diagnosis? A. B. C. D. E. Surgical lung biopsy 2 Transbronchial biopsy Serologies 1 Referral to rheumatology Diagnosis is clear, no more information is necessary
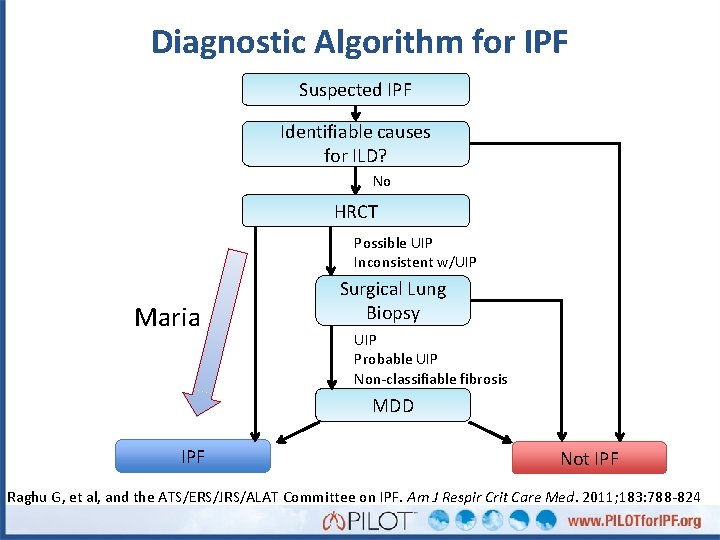
Diagnostic Algorithm for IPF Suspected IPF Identifiable causes for ILD? No HRCT Possible UIP Inconsistent w/UIP Maria Surgical Lung Biopsy UIP Probable UIP Non-classifiable fibrosis MDD IPF Not IPF Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
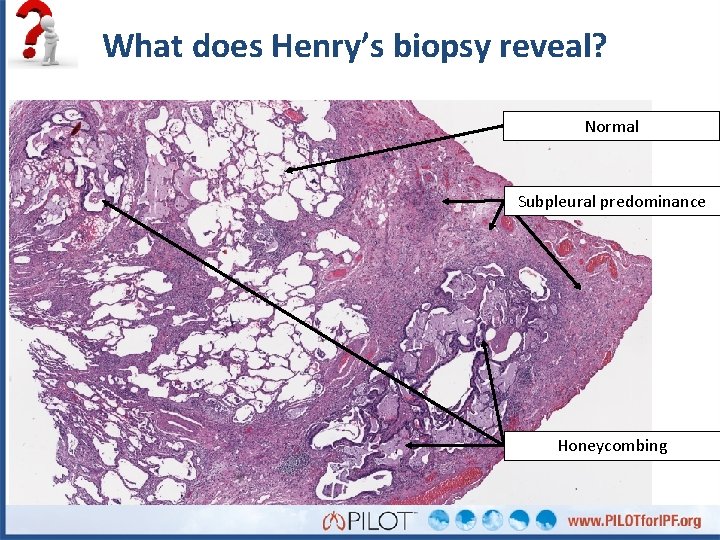
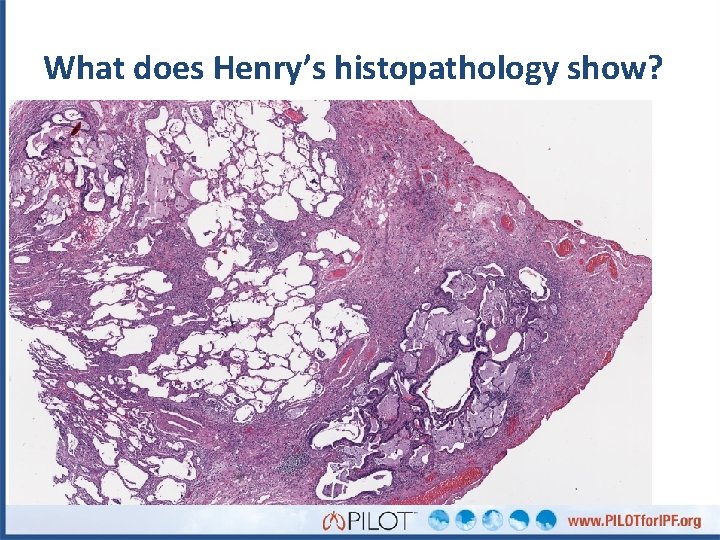
What does Henry’s biopsy reveal? Normal Subpleural predominance Honeycombing
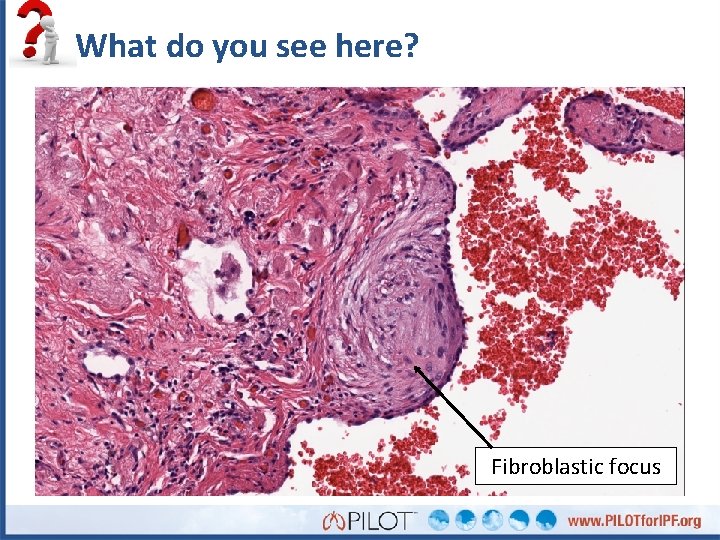
What do you see here? Fibroblastic focus
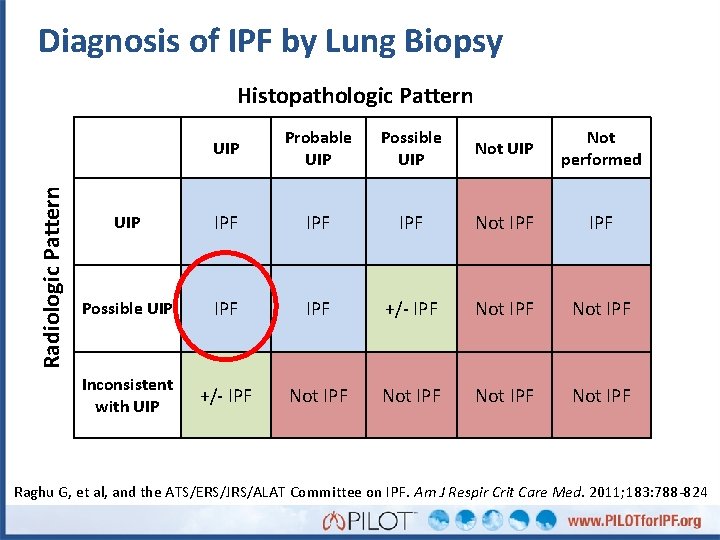
Diagnosis of IPF by Lung Biopsy Radiologic Pattern Histopathologic Pattern UIP Probable UIP Possible UIP Not performed UIP IPF IPF Not IPF Possible UIP IPF +/- IPF Not IPF Inconsistent with UIP +/- IPF Not IPF Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
Shared Decision-Making With Henry Physician • • • Drug Efficacy Side Effects Comorbidities • Preferences • Pill Burden Acceptance • Side Effect Tolerance

Which therapy would you recommend for Henry? • Henry expressed a preference for nintedanib given • • his regular sun exposure He was started on 150 mg 2 x daily After 2 weeks he complained of diarrhea Management: – Dose reduced to 100 mg 2 x daily with loperamide – Diarrhea subsided after 1 week – Dose escalated to 150 mg 2 x daily after 2 weeks Strategy was successful – No further side effects reported – No liver enzyme abnormalities
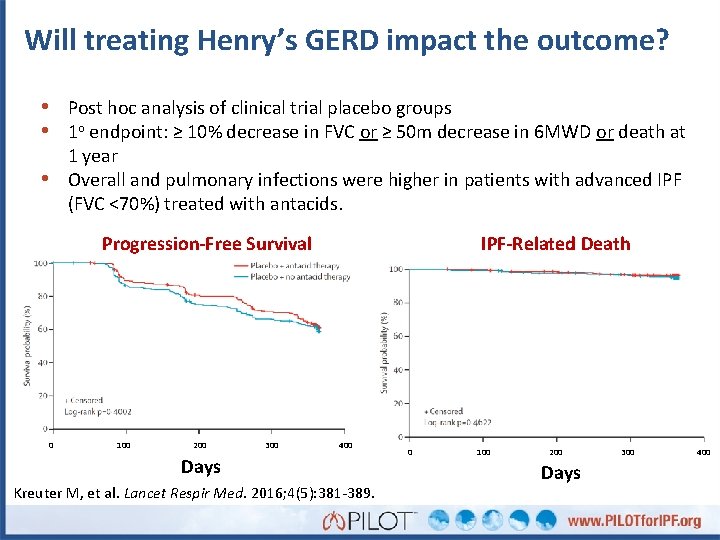
Will treating Henry’s GERD impact the outcome? • Post hoc analysis of clinical trial placebo groups • 1 o endpoint: ≥ 10% decrease in FVC or ≥ 50 m decrease in 6 MWD or death at 1 year Overall and pulmonary infections were higher in patients with advanced IPF (FVC <70%) treated with antacids. • Progression-Free Survival 0 100 200 300 IPF-Related Death 400 Days Kreuter M, et al. Lancet Respir Med. 2016; 4(5): 381 -389. 0 100 200 Days 300 400

Monitoring for Disease Progression • Every 3 months: – – – PFTs (at least FVC and DLCO) 6 MWT (distance/nadir saturation) O 2 requirement during activity Comorbidities Consider dyspnea questionnaire (UCSD) Consider periodic overnight pulse oximetry to assess for nocturnal desaturation • HRCT – Upon suspicion of clinical worsening
Henry Progresses Slowly • FVC continued to decline – 12 months: 76% – 24 months: 74% • HRCT shows progression • Henry reports that his SOB is “a little bit worse” • Important to manage patient expectations
Henry Summary • Possible UIP on HRCT requires histology for • • • IPF diagnosis Outdoor lifestyle and patient preference led to choice of nintedanib Common GI side effects managed with dose reduction and loperamide IPF progressed during therapy

Conclusions • Diagnosis – Histology is sometimes necessary to establish diagnosis • Treatment decisions depend on patient factors and preferences – Shared decision-making – Comorbidities can impact treatment choice • Pirfenidone and nintedanib slow disease progression – Side effects are usually manageable

Q&A • The 2 available therapies have similar efficacy. How • • • will you decide between them? How do you decide when to switch medications? How can you distinguish between treatment failure and disease progression in an individual patient? In what fraction of cases is the dose reduction protocol effective for each drug?

Questions? • Please fill out the posttest and evaluation (thank you) • Look for Case 3 online! • www. PILOTfor. IPF. org
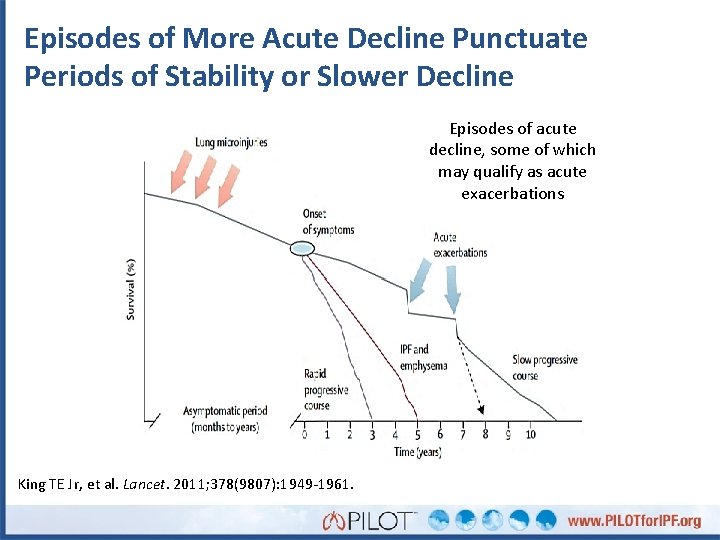
Episodes of More Acute Decline Punctuate Periods of Stability or Slower Decline Episodes of acute decline, some of which may qualify as acute exacerbations King TE Jr, et al. Lancet. 2011; 378(9807): 1949 -1961.

Maria • 69 -year-old white woman • Most recent job: line worker in auto assembly plant • • for 15 y Presentation – Progressive shortness of breath on exertion for 6 months – Dry hacking cough for 3 months – Complains of joint stiffness in morning Referred from primary care physician for further work-up
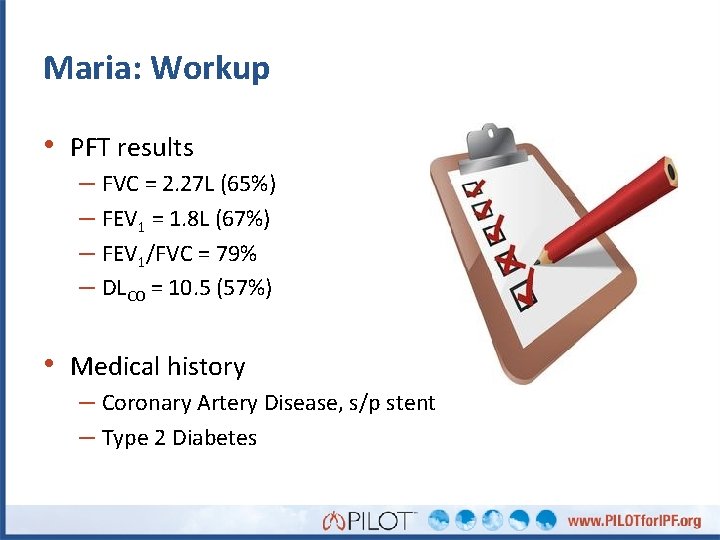
Maria: Workup • PFT results – FVC = 2. 27 L (65%) – FEV 1 = 1. 8 L (67%) – FEV 1/FVC = 79% – DLCO = 10. 5 (57%) • Medical history – Coronary Artery Disease, s/p stent – Type 2 Diabetes
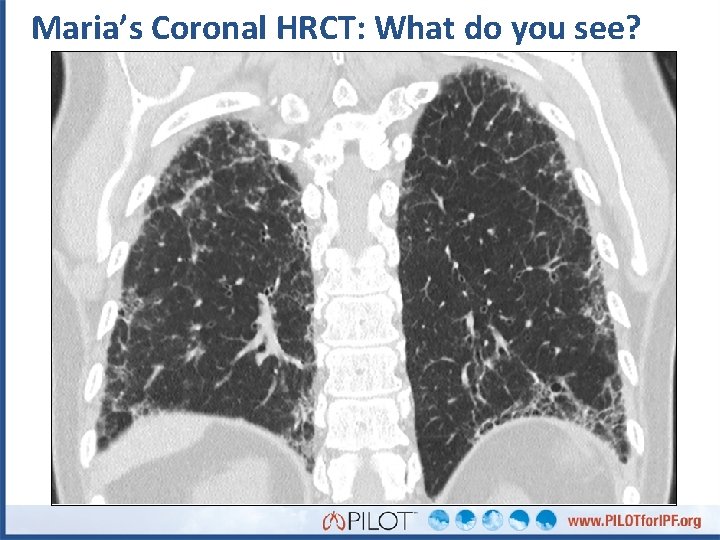
Maria’s Coronal HRCT: What do you see?
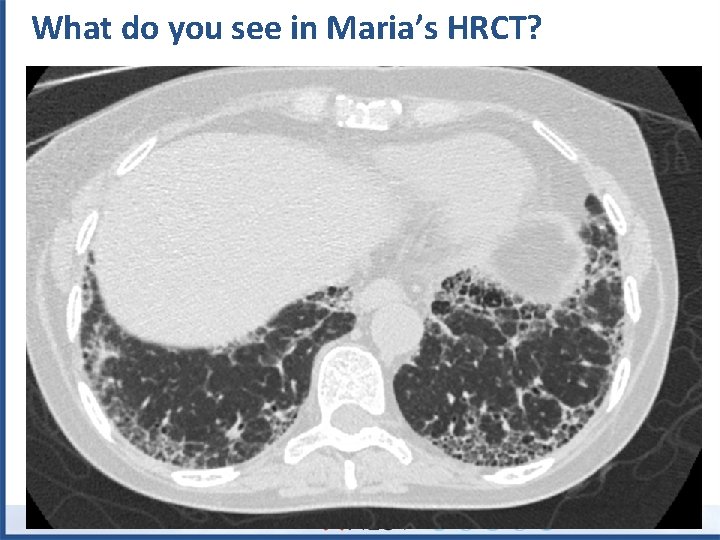
What do you see in Maria’s HRCT?
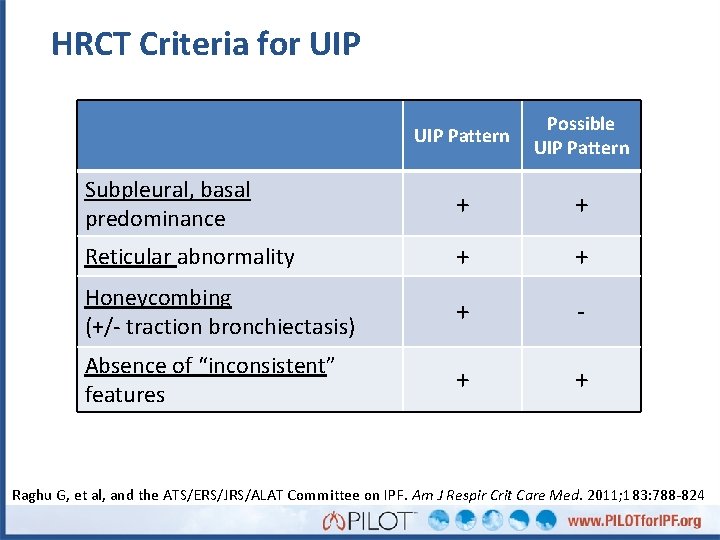
HRCT Criteria for UIP Pattern Possible UIP Pattern Subpleural, basal predominance + + Reticular abnormality + + Honeycombing (+/- traction bronchiectasis) + - Absence of “inconsistent” features + + Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
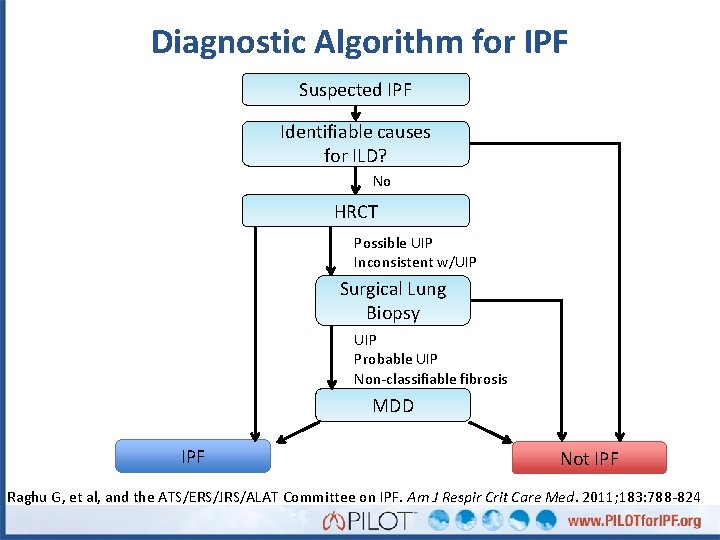
Diagnostic Algorithm for IPF Suspected IPF Identifiable causes for ILD? No HRCT Possible UIP Inconsistent w/UIP Surgical Lung Biopsy UIP Probable UIP Non-classifiable fibrosis MDD IPF Not IPF Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
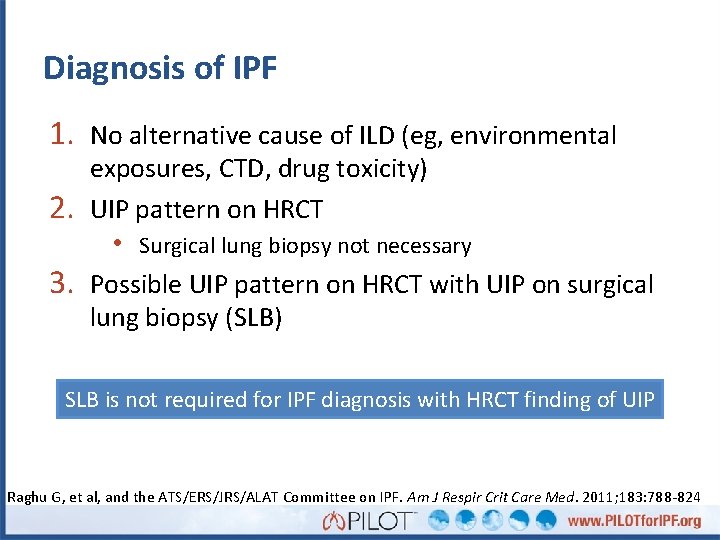
Diagnosis of IPF 1. No alternative cause of ILD (eg, environmental 2. 3. exposures, CTD, drug toxicity) UIP pattern on HRCT • Surgical lung biopsy not necessary Possible UIP pattern on HRCT with UIP on surgical lung biopsy (SLB) SLB is not required for IPF diagnosis with HRCT finding of UIP Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824

Diagnostic Paradigm Clinical Serology Radiology IPF Environmental History
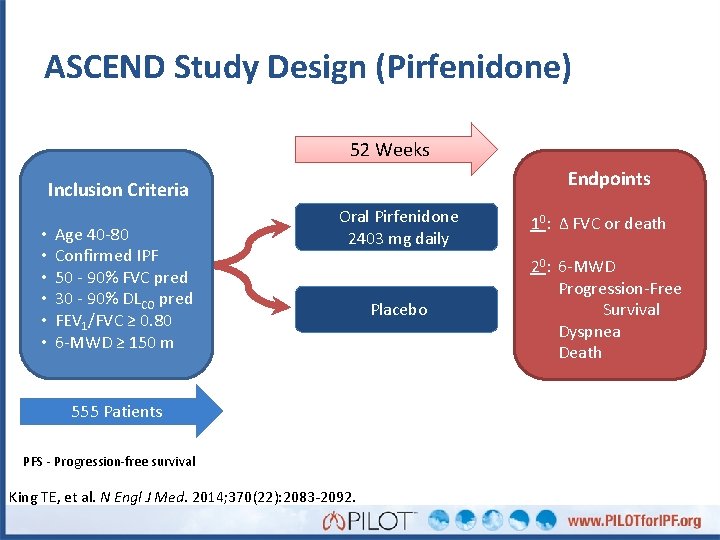
ASCEND Study Design (Pirfenidone) 52 Weeks Endpoints Inclusion Criteria • • • Age 40 -80 Confirmed IPF 50 - 90% FVC pred 30 - 90% DLCO pred FEV 1/FVC ≥ 0. 80 6 -MWD ≥ 150 m Oral Pirfenidone 2403 mg daily 555 Patients PFS - Progression-free survival King TE, et al. N Engl J Med. 2014; 370(22): 2083 -2092. Placebo 10: Δ FVC or death 20: 6 -MWD Progression-Free Survival Dyspnea Death
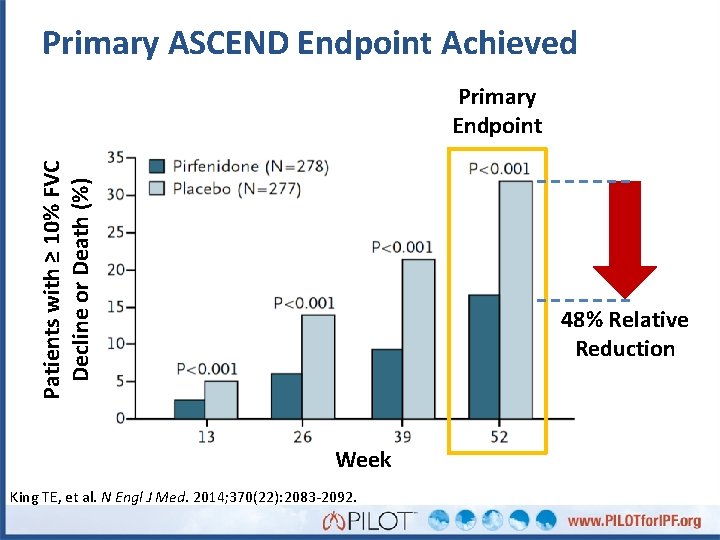
Primary ASCEND Endpoint Achieved Patients with ≥ 10% FVC Decline or Death (%) Primary Endpoint 48% Relative Reduction Week King TE, et al. N Engl J Med. 2014; 370(22): 2083 -2092.
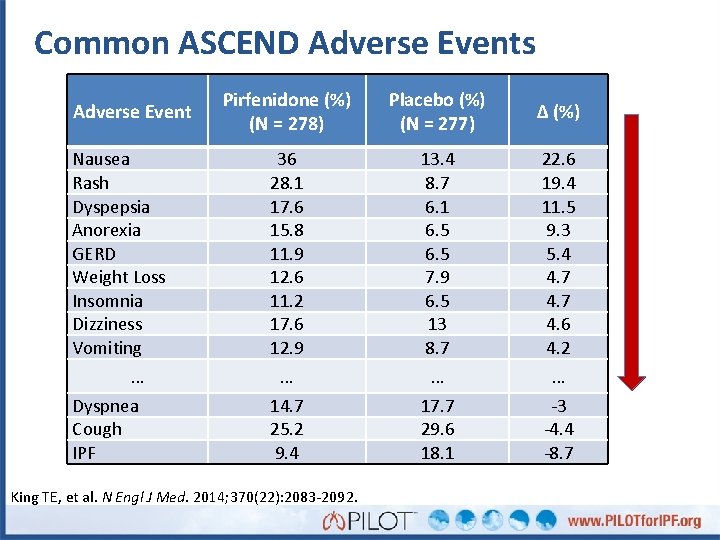
Common ASCEND Adverse Events Adverse Event Nausea Rash Dyspepsia Anorexia GERD Weight Loss Insomnia Dizziness Vomiting … Dyspnea Cough IPF Pirfenidone (%) (N = 278) Placebo (%) (N = 277) Δ (%) 36 28. 1 17. 6 15. 8 11. 9 12. 6 11. 2 17. 6 12. 9 13. 4 8. 7 6. 1 6. 5 7. 9 6. 5 13 8. 7 22. 6 19. 4 11. 5 9. 3 5. 4 4. 7 4. 6 4. 2 … … … 14. 7 25. 2 9. 4 17. 7 29. 6 18. 1 -3 -4. 4 -8. 7 King TE, et al. N Engl J Med. 2014; 370(22): 2083 -2092.
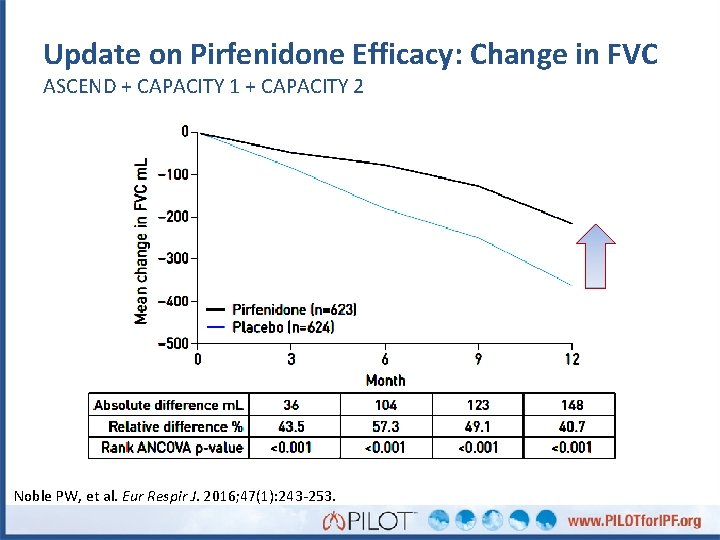
Update on Pirfenidone Efficacy: Change in FVC ASCEND + CAPACITY 1 + CAPACITY 2 Noble PW, et al. Eur Respir J. 2016; 47(1): 243 -253.
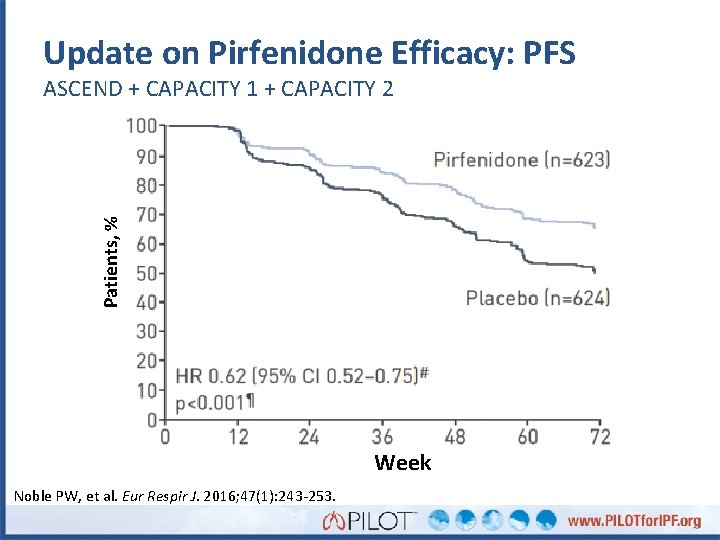
Update on Pirfenidone Efficacy: PFS Patients, % ASCEND + CAPACITY 1 + CAPACITY 2 Week Noble PW, et al. Eur Respir J. 2016; 47(1): 243 -253.
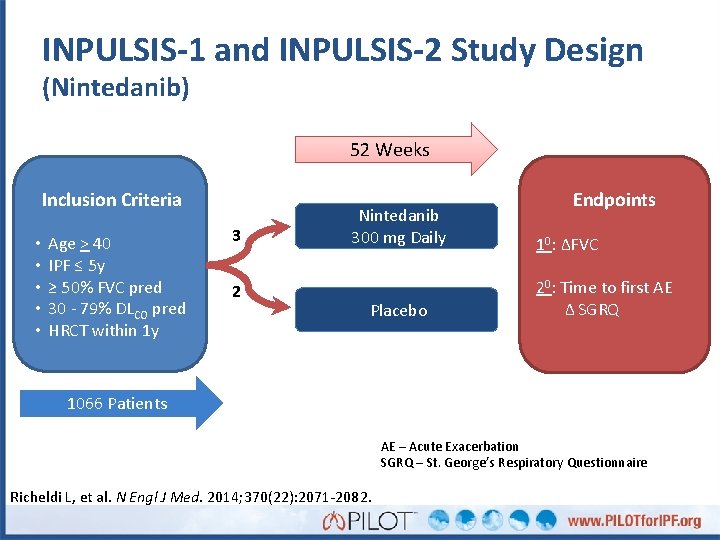
INPULSIS-1 and INPULSIS-2 Study Design (Nintedanib) 52 Weeks Inclusion Criteria • • • Age > 40 IPF ≤ 5 y ≥ 50% FVC pred 30 - 79% DLCO pred HRCT within 1 y 3 2 Nintedanib 300 mg Daily Placebo Endpoints 10: ΔFVC 20: Time to first AE Δ SGRQ 1066 Patients AE – Acute Exacerbation SGRQ – St. George’s Respiratory Questionnaire Richeldi L, et al. N Engl J Med. 2014; 370(22): 2071 -2082.
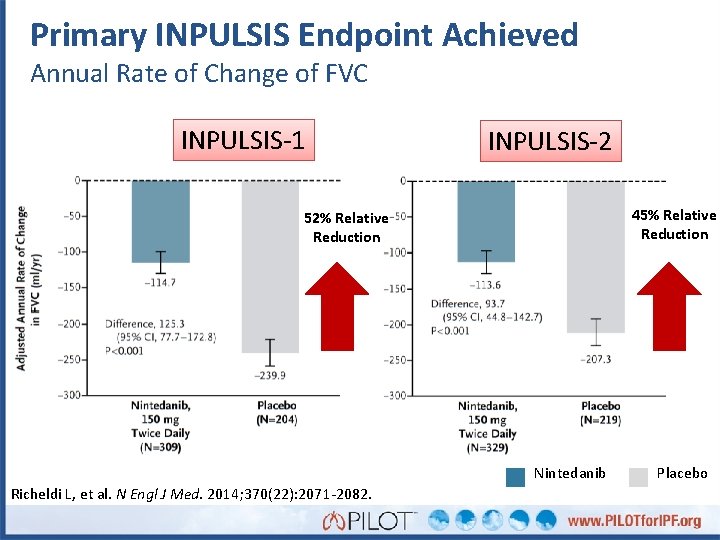
Primary INPULSIS Endpoint Achieved Annual Rate of Change of FVC INPULSIS-1 INPULSIS-2 45% Relative Reduction 52% Relative Reduction Nintedanib Richeldi L, et al. N Engl J Med. 2014; 370(22): 2071 -2082. Placebo
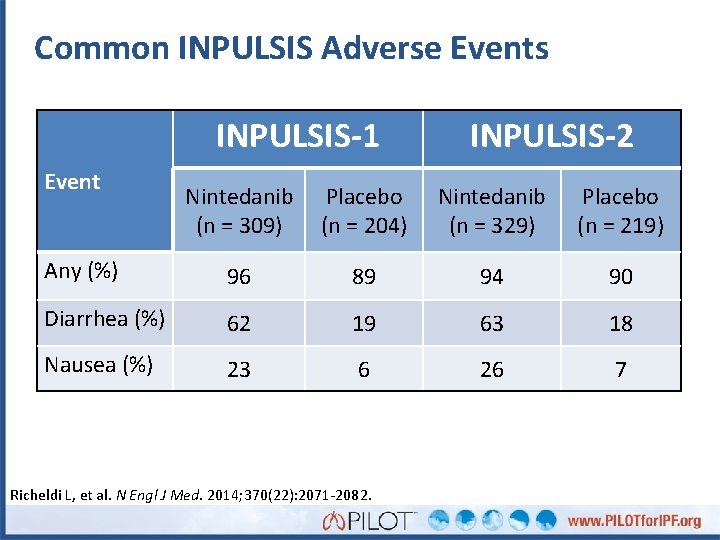
Common INPULSIS Adverse Events INPULSIS-1 Event INPULSIS-2 Nintedanib (n = 309) Placebo (n = 204) Nintedanib (n = 329) Placebo (n = 219) Any (%) 96 89 94 90 Diarrhea (%) 62 19 63 18 Nausea (%) 23 6 26 7 Richeldi L, et al. N Engl J Med. 2014; 370(22): 2071 -2082.
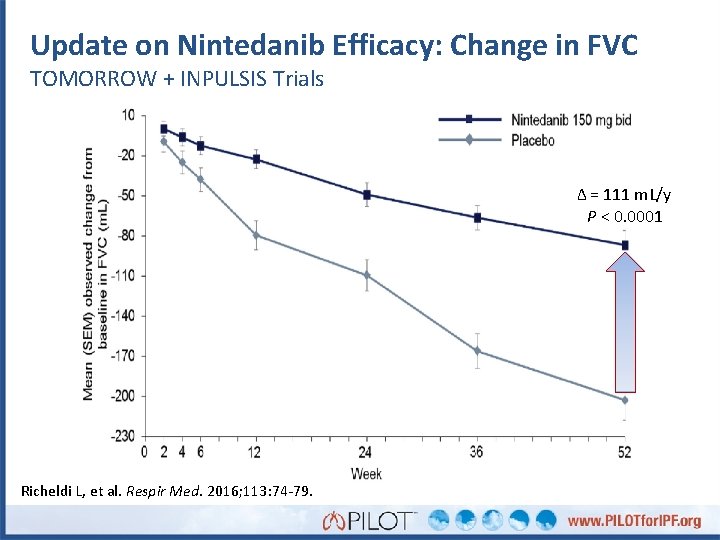
Update on Nintedanib Efficacy: Change in FVC TOMORROW + INPULSIS Trials Δ = 111 m. L/y P < 0. 0001 Richeldi L, et al. Respir Med. 2016; 113: 74 -79.
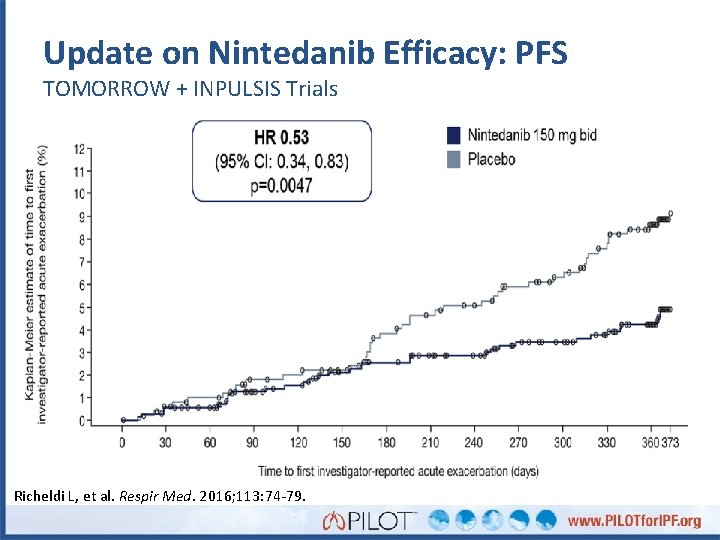
Update on Nintedanib Efficacy: PFS TOMORROW + INPULSIS Trials Richeldi L, et al. Respir Med. 2016; 113: 74 -79.
Maria is concerned about an acute exacerbation • Acute respiratory decline • ~5– 10% of patients/year • Criteria – Unexplained worsening of dyspnea within 1 month – Evidence of hypoxemia as defined by worsened or severely impaired gas exchange – New radiographic alveolar infiltrates – Absence of an alternative explanation such as • Infection • Pulmonary embolism • Pneumothorax or • Heart failure Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
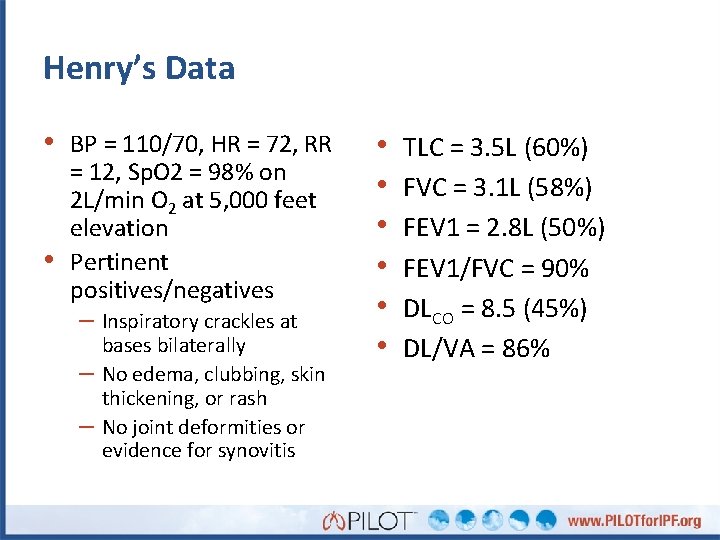
Henry’s Data • BP = 110/70, HR = 72, RR • = 12, Sp. O 2 = 98% on 2 L/min O 2 at 5, 000 feet elevation Pertinent positives/negatives – Inspiratory crackles at – – bases bilaterally No edema, clubbing, skin thickening, or rash No joint deformities or evidence for synovitis • • • TLC = 3. 5 L (60%) FVC = 3. 1 L (58%) FEV 1 = 2. 8 L (50%) FEV 1/FVC = 90% DLCO = 8. 5 (45%) DL/VA = 86%
Chest HRCT
What does Henry’s histopathology show?
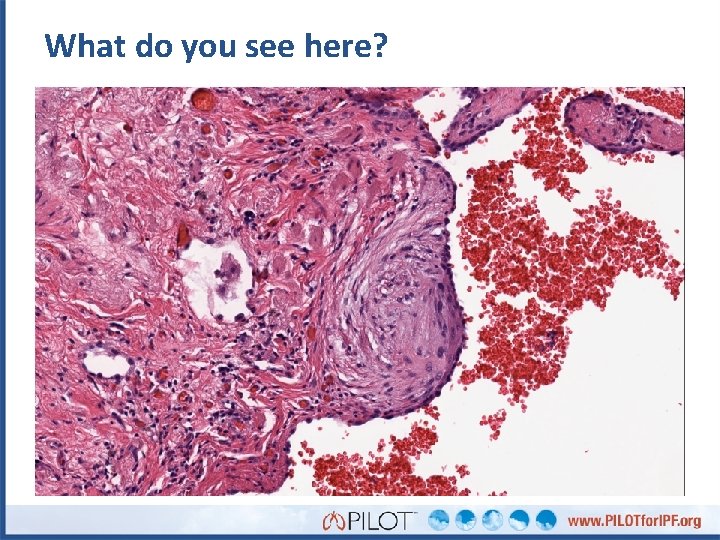
What do you see here?
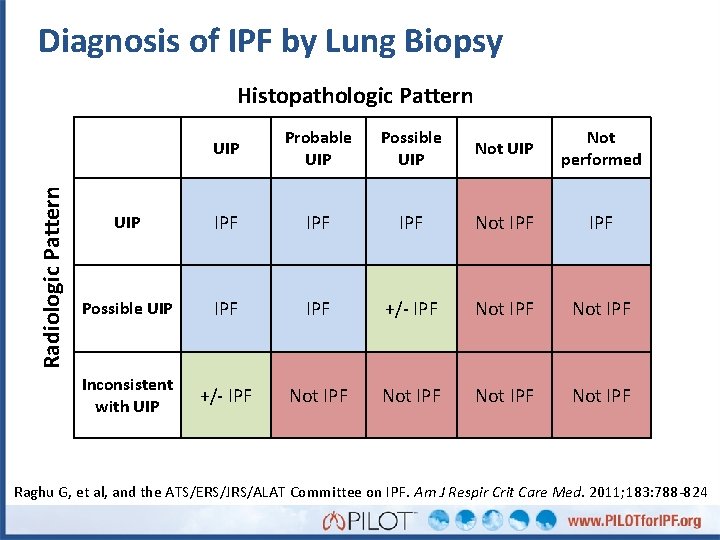
Diagnosis of IPF by Lung Biopsy Radiologic Pattern Histopathologic Pattern UIP Probable UIP Possible UIP Not performed UIP IPF IPF Not IPF Possible UIP IPF +/- IPF Not IPF Inconsistent with UIP +/- IPF Not IPF Raghu G, et al, and the ATS/ERS/JRS/ALAT Committee on IPF. Am J Respir Crit Care Med. 2011; 183: 788 -824
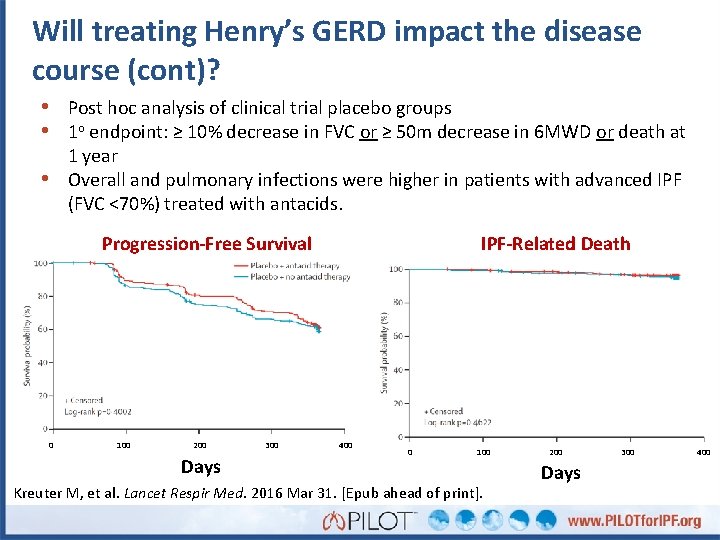
Will treating Henry’s GERD impact the disease course (cont)? • Post hoc analysis of clinical trial placebo groups • 1 o endpoint: ≥ 10% decrease in FVC or ≥ 50 m decrease in 6 MWD or death at 1 year Overall and pulmonary infections were higher in patients with advanced IPF (FVC <70%) treated with antacids. • Progression-Free Survival 0 100 200 Days 300 IPF-Related Death 400 0 100 Kreuter M, et al. Lancet Respir Med. 2016 Mar 31. [Epub ahead of print]. 200 Days 300 400

Monitoring for Disease Progression • Every 3 months: – – – PFTs (at least FVC and DLCO) 6 MWT (distance/nadir saturation) O 2 requirement during activity Comorbidities Consider dyspnea questionnaire (UCSD) Consider periodic overnight pulse oximetry to assess for nocturnal desaturation • HRCT – Upon suspicion of clinical worsening

IPF Resources on PILOTfor. IPF. org üIPF Management Checklist üHRCT criteria for UIP üDiagnostic algorithm üDiagnosis with radiology and histology criteria üMonitoring recommendations
- Slides: 82