BRAZING FLUX STUDIES INTERNATIONAL BRAZING AND SOLDERING CONFERENCE

BRAZING FLUX STUDIES INTERNATIONAL BRAZING AND SOLDERING CONFERENCE ALBUQUERQUE, NEW MEXICO / APRIL 4, 2000 s Presented By: Dr. Y. Baskin Superior Flux & Manufacturing Company Cleveland, Ohio SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000

PURPOSE s To study the effects of time, temperature, and flux formulation on activation and exhaustion temperatures, which determine flux activity ranges. The role of several different base metals and filler metals was also studied SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000

EQUIPMENT s s s Thermolyne 1500 Furnace - Maximum Temperature 1300° C 30 Tempilstik Temperature Indicators 400° - 1200° C Calibrated Pyrometer with Surface Probe Timer Balance SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000

BASE METALS s s Mild Steel Stainless Steel 316 Copper Brass (70% Copper, 30% Zinc) SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000

FILLER METALS Bag-1 s Composition: 45% Silver, 15% Copper, 16% Zinc, 24% Cadmium Melting Point: 618° C Braze 630 - Bag-21, SAE-AMS 4774 s Composition: 63% Silver, 28. 5% Copper, 6% Tin, 2. 5% Nickel Melting Point: 690° C High-Temp 095, SAE-AMS 4764 s Composition: 52. 5% Copper, 38% Manganese, 9. 5% Nickel Melting Point: 875° C SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000

FLUXES Fifteen fluxes were used, including existing products and experimental, formulations. Compositions included the following raw materials: s Boric Acid s Potassium Bifluoride s Potassium Tetraborate s Sodium Tetraborate (Borax) s Potassium Fluoborate s Silica s Potassium Carbonate s Potassium Pentaborate s Potassium Fluroide SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000
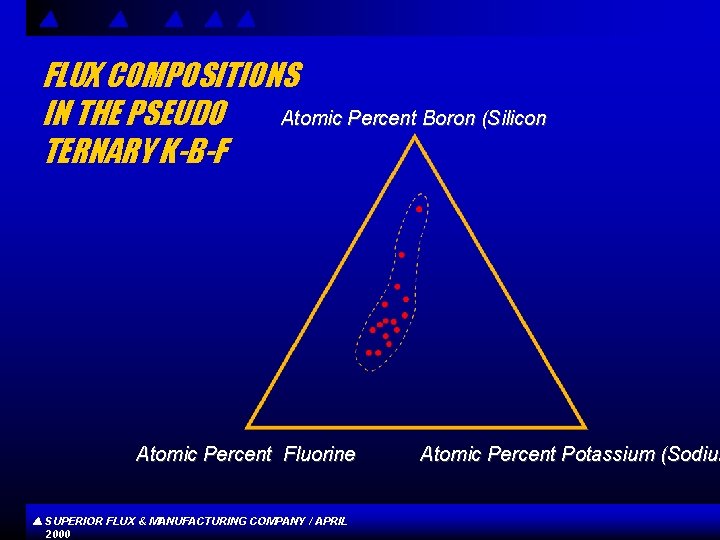
FLUX COMPOSITIONS IN THE PSEUDO Atomic Percent Boron (Silicon TERNARY K-B-F Atomic Percent Fluorine SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000 Atomic Percent Potassium (Sodium
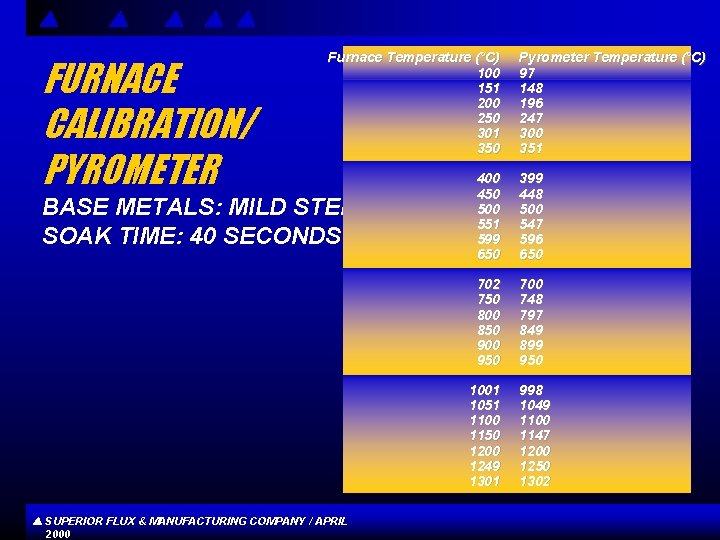
FURNACE CALIBRATION/ PYROMETER Furnace Temperature (°C) 100 151 200 250 301 350 BASE METALS: MILD STEEL SOAK TIME: 40 SECONDS SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000 Pyrometer Temperature (°C) 97 148 196 247 300 351 400 450 500 551 599 650 399 448 500 547 596 650 702 750 800 850 900 950 700 748 797 849 899 950 1001 1051 1100 1150 1200 1249 1301 998 1049 1100 1147 1200 1250 1302
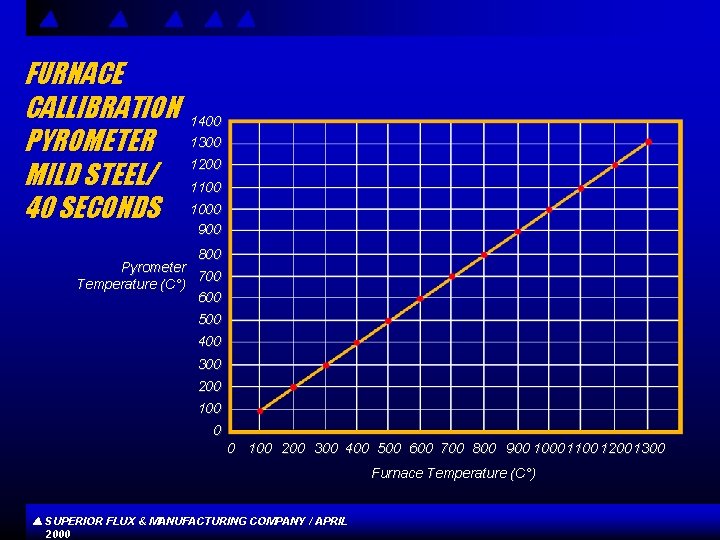
FURNACE CALLIBRATION PYROMETER MILD STEEL/ 40 SECONDS 1400 1300 1200 1100 1000 900 800 Pyrometer 700 Temperature (C°) 600 500 400 300 200 100 0 0 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 Furnace Temperature (C°) SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000
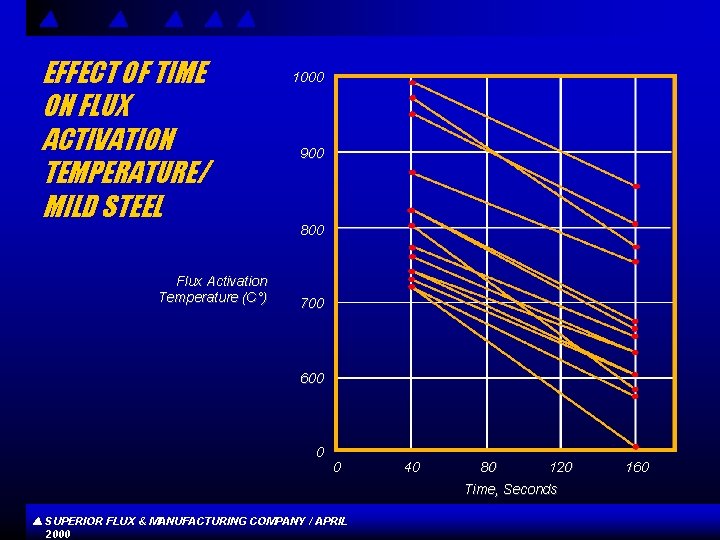
EFFECT OF TIME ON FLUX ACTIVATION TEMPERATURE/ MILD STEEL Flux Activation Temperature (C°) 1000 900 800 700 600 0 0 40 80 120 Time, Seconds SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000 160
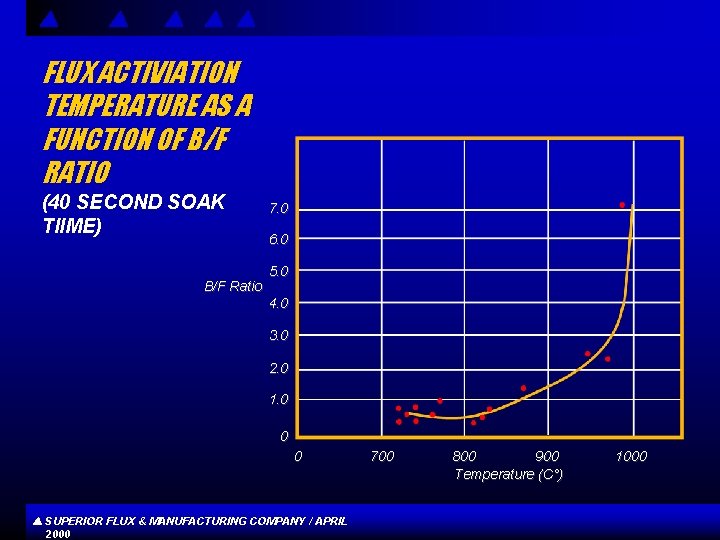
FLUX ACTIVIATION TEMPERATURE AS A FUNCTION OF B/F RATIO (40 SECOND SOAK TIIME) B/F Ratio 7. 0 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0 0 SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000 700 800 900 Temperature (C°) 1000
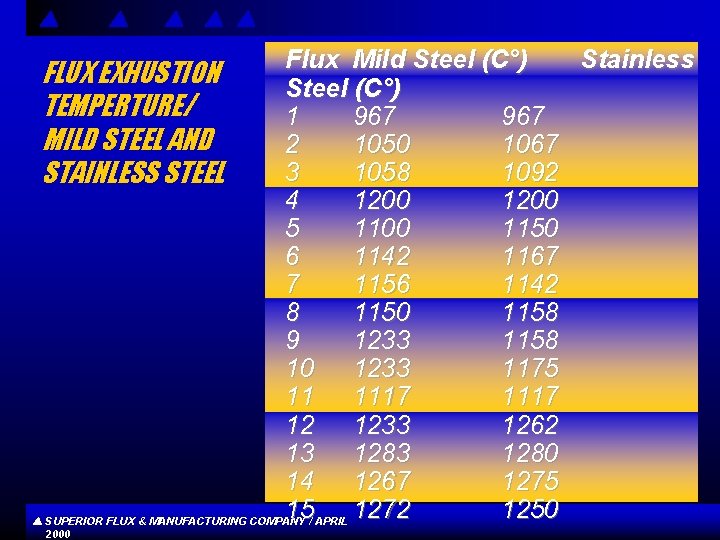
Flux Mild Steel(C°) Stainless Steel (C°) 1 967 2 1050 1067 3 1058 1092 4 1200 5 1100 1150 6 1142 1167 7 1156 1142 8 1150 1158 9 1233 1158 10 1233 1175 11 1117 12 1233 1262 13 1280 14 1267 1275 15 1272 1250 SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL FLUX EXHUSTION TEMPERTURE/ MILD STEEL AND STAINLESS STEEL 2000
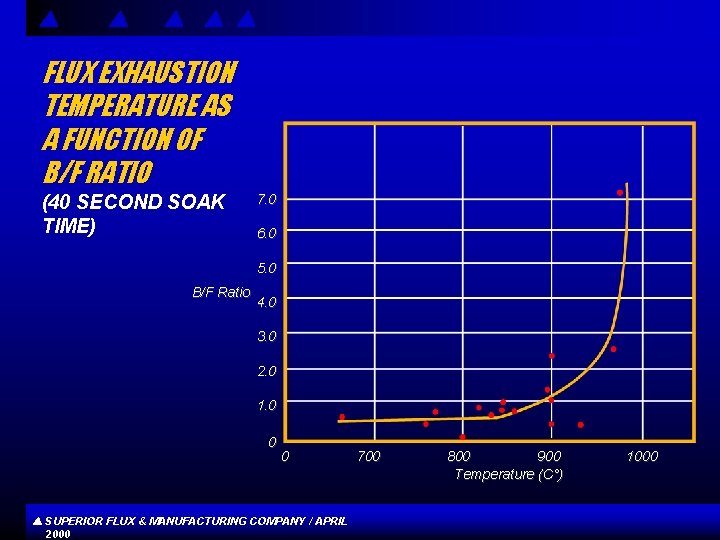
FLUX EXHAUSTION TEMPERATURE AS A FUNCTION OF B/F RATIO (40 SECOND SOAK TIME) 7. 0 6. 0 5. 0 B/F Ratio 4. 0 3. 0 2. 0 1. 0 0 0 SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000 700 800 900 Temperature (C°) 1000

CONCLUSIONS s s Flux compositions high in boron and low in fluorine generally exhibit better high temperature properties , whereas compositions low in boron and high in fluorine show better and more active low temperature properties, Difference s in raw materials and other chemical factors may account for the departure from a more linear relationship between activation or exhaustion temperature and B/F ratios. Increased soaking time reduces flux activation temperature. SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000

CONCLUSIONS (Continued) s s Neither flux activation temperature nor exhaustion temperature affected by the base metal used, as long as the stability range of the metal is not exceeded. Similarly flux exhaustion temperatures are not affected by the filler metal used, as long as the stability of the filler metal is not exceeded. SUPERIOR FLUX & MANUFACTURING COMPANY / APRIL 2000
- Slides: 15