Boyles Law Charles Law and Gay Lussacs Law

Boyle’s Law, Charles’ Law, and Gay. Lussac’s Law

� Kinetic Molecular Theory- particles are in constant random motion � Kinetic theory of gases- we can assume these 4 things � 1 - gas consists of a large # of particles in constant random motion � 2 - Gas molecules influence each other by collision- have no forces between each other to keep them together � 3 - All energy is conserved when particles hit each other (energy does not change) � 4 - the volume occupied by gases is very smallthe majority of a gases volume is empty space

� The force that the particles of a gas exert on the walls of their container �Also called “force per unit area” � Which �The ball has a higher pressure? Why? one that is NOT deflated �As more air is pumped into the basketball the number of gas particles inside the ball increases. �This means that more particles bounce off of and push against the inside walls of the basketball
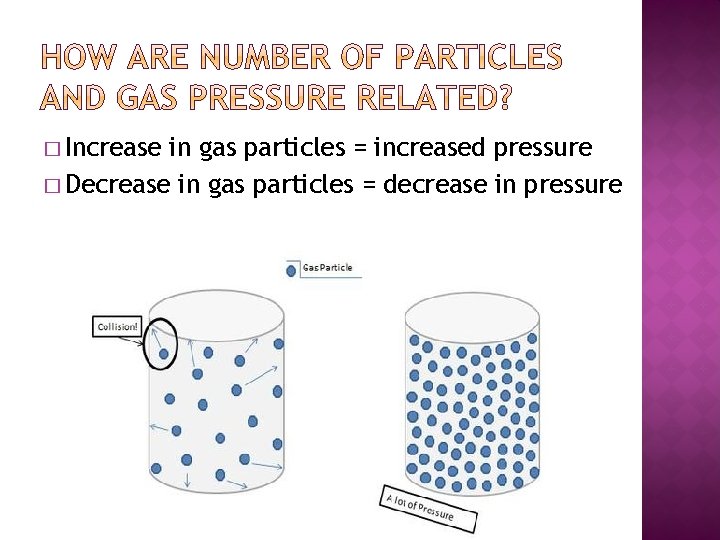
� Increase in gas particles = increased pressure � Decrease in gas particles = decrease in pressure
� Barometer �A piece of equipment used to measure gas pressure �Measures the amount of pressure exerted by the atmosphere

Pressure (P): force per unit area � 1 atm= � 760 mm Hg= � 101. 325 k. Pa= � 101, 325 pascals= � 14. 7 psi=

� Example �In 1 weather reports, barometric pressure is often expressed in inches of mercury. What is one standard atmosphere express in inches of mercury? (1 in = 25. 4 mm)

� Example �The 2 reading on a tire gauge is 35 psi. What is the equivalent pressure in kilopascals?

� Example �Convert 3 1140 mm HG to k. Pa
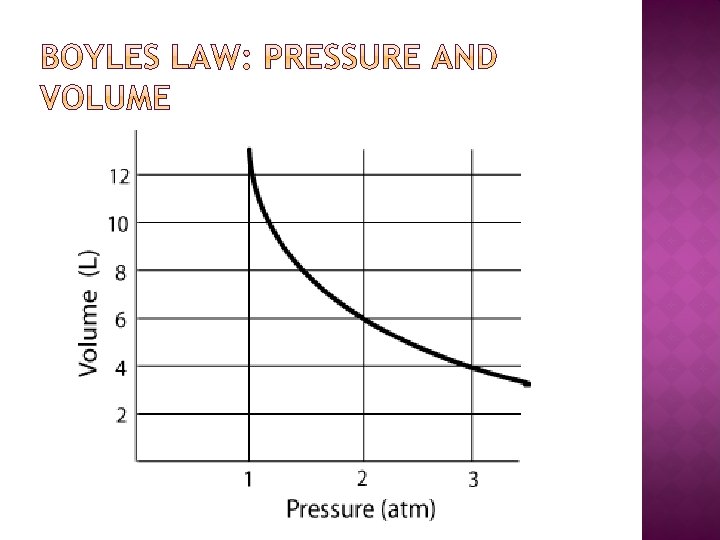
� At constant temperature, if the pressure of a gas increases, its volume decreases � An inverse relationship �This means that as one variable increases, the other decreases � Think about what happens when you apply different amounts of pressure to the same object. � We can express the relationship between pressure and volume with an equation �P 1 V 1= P 2 V 2

� https: //www. youtube. com/watch? v=27 yq. J 9 v J 5 k. Q

� Example �A 1: high altitude balloon contains 30. 0 L of helium gas at 103 kpa. What is the volume in liters when the balloon rises to an altitude where the pressure is only 25. 0 kpa? (assume that the temperature remains constant. )

� The pressure on 2. 50 L of anesthetic gas changes from 105 k. Pa to 40. 5 Kpa. What will be the new volume in m. L if the temperature remains constant?

�A gas with a volume of 4. 00 L at a pressure of 205 kpa is allowed to expand to a volume of 12. 0 L. What is the pressure in the container if the temperature remains constant? � When you think you have it come get my initials!

� Volume of a gas is directly proportional to the temperature @ constant pressure and moles. � What does directly proportional mean? � V 1/T 1= V 2/T 2 � Temperature must always be expressed in Kelvin when you plug it into this equation �K = C + 273 �C = K - 273

� Example �A 1: balloon inflated in a room at 24 degrees C has a volume of 4. 00 L. The balloon is then heated to a temperature of 58 degrees C. What is the new volume in liters if the pressure remains constant?

� If a sample of gas occupies 6. 80 L at 35 degrees C, what will be its volume at 25 degrees C if the pressure does not change?

� Exactly 5. 00 L of air at 0 degrees C is expanded to 10 L of air. What is the new temperature if the pressure remains constant? � **When you’re finished please raise your hand have me come check it for extra bell ringer points **
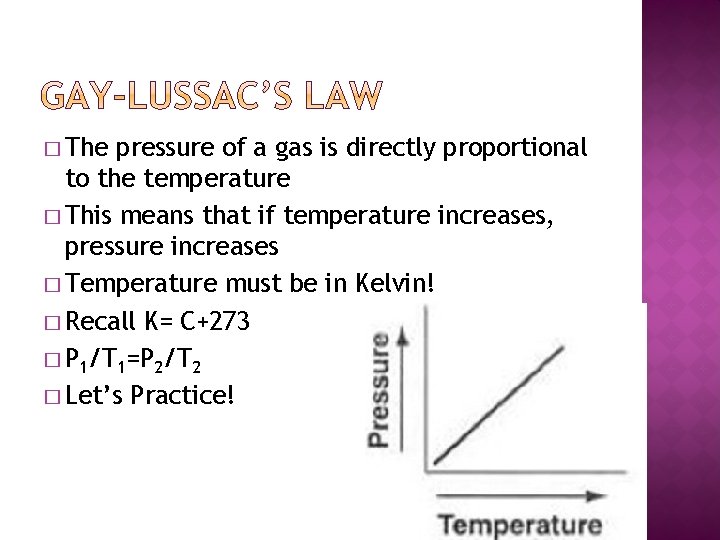
� The pressure of a gas is directly proportional to the temperature � This means that if temperature increases, pressure increases � Temperature must be in Kelvin! � Recall K= C+273 � P 1/T 1=P 2/T 2 � Let’s Practice!

� The gas left in a used aerosol can is at a pressure of 103 kpa at 300 degrees K. If this can is thrown onto a fire, what is the pressure of the gas when its temperature reaches 938 degrees k?

�A gas has a pressure of 6. 58 kpa at 539 K. What will be the pressure at 211 K if the volume does not change?

� The pressure in an automobile tire is 198 kpa at 27 degrees C. At the end of a trip on a hot sunny day, the pressure has risen to 225 kpa. What is the temperature of the air in the tire? � Now, why do more tire blow outs in the summer time?
- Slides: 23