Boyles Gas Law At constant temperature and quantity
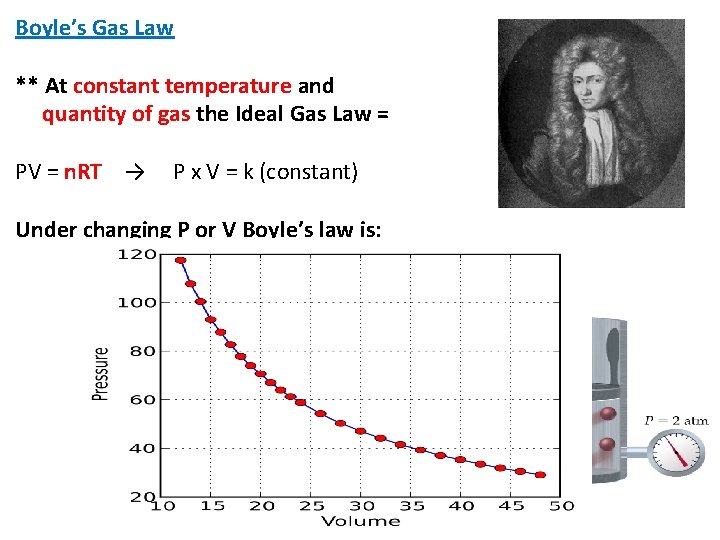
Boyle’s Gas Law ** At constant temperature and quantity of gas the Ideal Gas Law = PV = n. RT → P x V = k (constant) Under changing P or V Boyle’s law is: P 1 V 1 = P 2 V 2
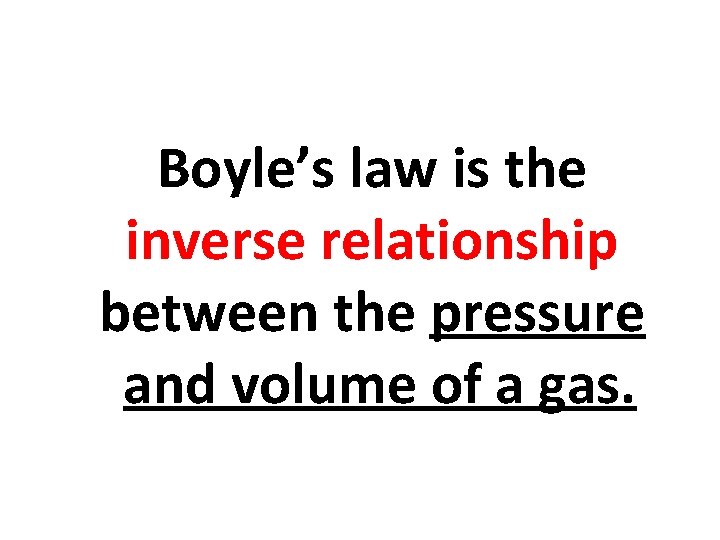
Boyle’s law is the inverse relationship between the pressure and volume of a gas.
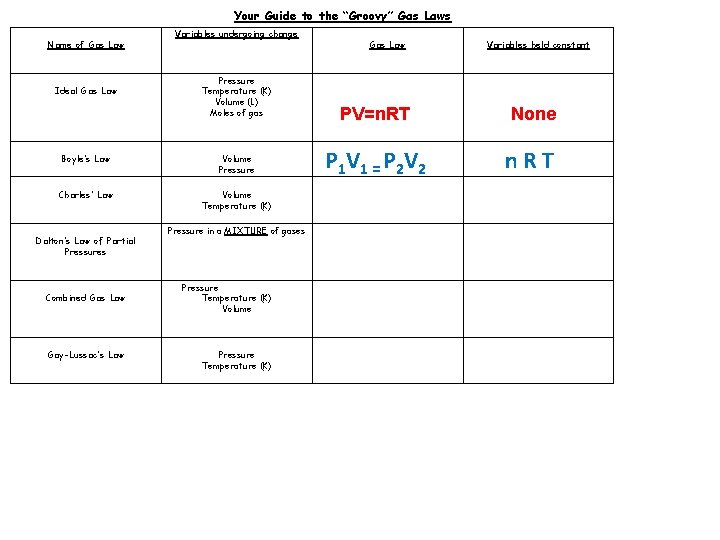
Your Guide to the “Groovy” Gas Laws Name of Gas Law Ideal Gas Law Variables undergoing change Pressure Temperature (K) Volume (L) Moles of gas Boyle’s Law Volume Pressure Charles’ Law Volume Temperature (K) Dalton’s Law of Partial Pressures Combined Gas Law Gay-Lussac’s Law Pressure in a MIXTURE of gases Pressure Temperature (K) Volume Pressure Temperature (K) Gas Law Variables held constant PV=n. RT None P 1 V 1 = P 2 V 2 n. RT
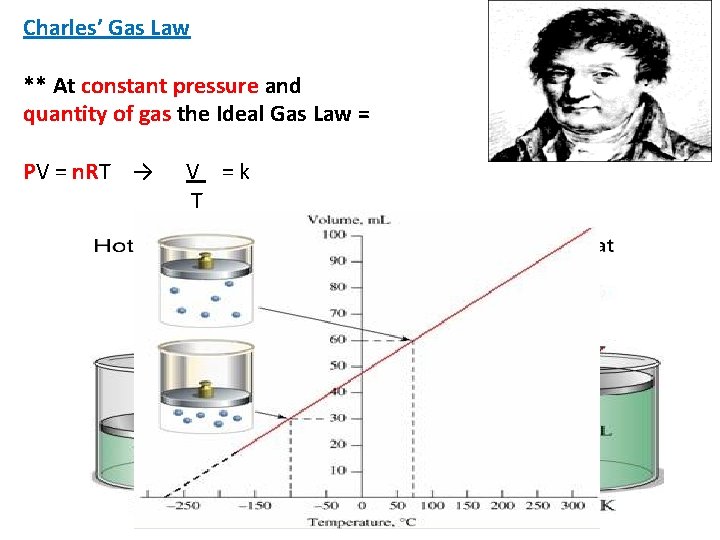
Charles’ Gas Law ** At constant pressure and quantity of gas the Ideal Gas Law = PV = n. RT → V =k T
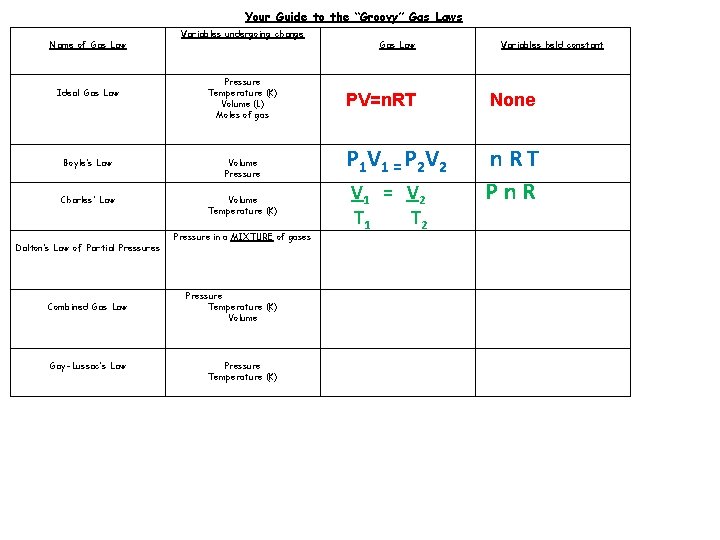
Your Guide to the “Groovy” Gas Laws Name of Gas Law Ideal Gas Law Variables undergoing change Pressure Temperature (K) Volume (L) Moles of gas Boyle’s Law Volume Pressure Charles’ Law Volume Temperature (K) Dalton’s Law of Partial Pressures Combined Gas Law Gay-Lussac’s Law Pressure in a MIXTURE of gases Pressure Temperature (K) Volume Pressure Temperature (K) Gas Law Variables held constant PV=n. RT None P 1 V 1 = P 2 V 2 n. RT Pn. R V 1 = V 2 T 1 T 2
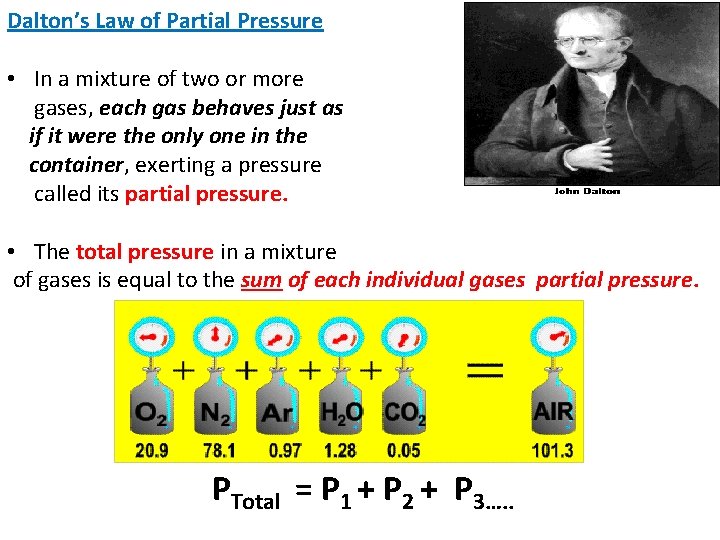
Dalton’s Law of Partial Pressure • In a mixture of two or more gases, each gas behaves just as if it were the only one in the container, exerting a pressure called its partial pressure. • The total pressure in a mixture of gases is equal to the sum of each individual gases partial pressure. PTotal = P 1 + P 2 + P 3…. .
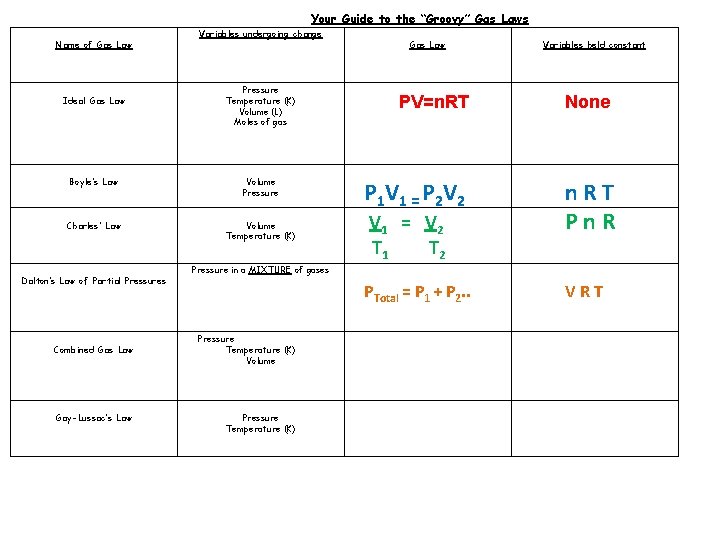
Your Guide to the “Groovy” Gas Laws Name of Gas Law Ideal Gas Law Variables undergoing change Pressure Temperature (K) Volume (L) Moles of gas Boyle’s Law Volume Pressure Charles’ Law Volume Temperature (K) Dalton’s Law of Partial Pressures Combined Gas Law Gay-Lussac’s Law Gas Law Variables held constant PV=n. RT None P 1 V 1 = P 2 V 2 n. RT Pn. R PTotal = P 1 + P 2. . VRT V 1 = V 2 T 1 T 2 Pressure in a MIXTURE of gases Pressure Temperature (K) Volume Pressure Temperature (K)
- Slides: 7