BONDING WHAT HOLDS THESE ELEMENTS TOGETHER Unit 2
BONDING: WHAT HOLDS THESE ELEMENTS TOGETHER? Unit 2: MOLECULES AND SOLUTIONS
Molecule A molecule is a group of two or more chemicaly bonded atoms.

Bonding Atoms do not bond at random. q Atoms want to be stable. (like a noble gas) q Goal = complete the last shell! q

Noble gas – Inert – STABLE! The last shell is complete!

Why do atoms Bond? Stability! If lithium loses its one valence electron then it will have two electrons in its outer shell. It will be stable as it resembles He, its closest noble gas. If chlorine gains one valence electron then it will have 8 electrons in its outer shell. It will be stable as it resembles Ar, its closest noble gas.

Chemical Stability Octet Rule: An atom with less than 8 electrons in the last shell tends to combine with another atom to fill its outer shell to a maximum of 8 electrons. A few exceptions exist where an atom requires only 2 valence electrons. (He and H) – Duet rule To become stable, some atoms will lose or gain electrons. Other atoms will share electrons with another atom.
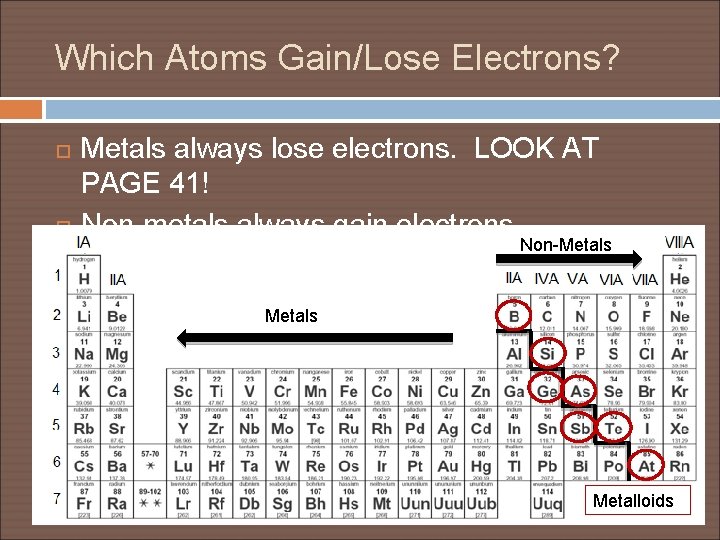
Which Atoms Gain/Lose Electrons? Metals always lose electrons. LOOK AT PAGE 41! Non-metals always gain electrons. Non-Metals Metalloids

What happens when an atom loses/gains an electron? Atoms are naturally neutral (#protons = #electrons) If electrons are gained or removed then the atom will have a charge. A charged atom is called an ion. If an atom gains electrons it becomes negatively charged. If an atom loses electrons it becomes positively charged. A positive ion is called a cation. (Mg 2+) A negative ion is called an anion. (Cl-)
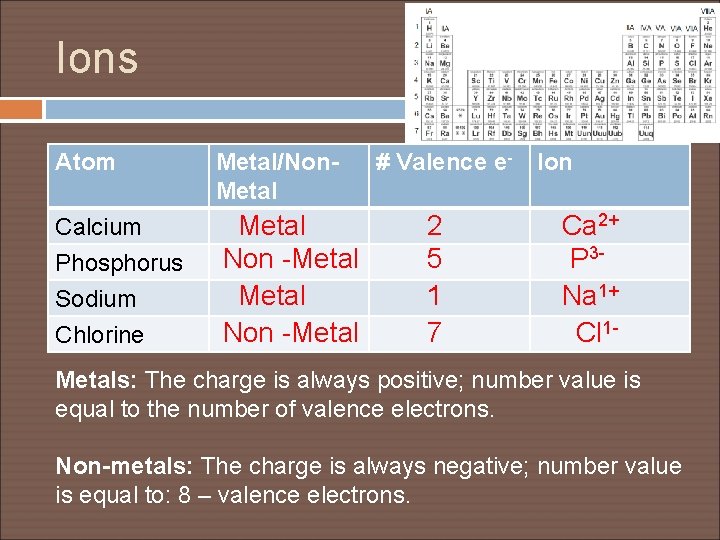
Ions Atom Metal/Non. Metal Calcium Phosphorus Sodium Chlorine Metal Non -Metal # Valence e- 2 5 1 7 Ion Ca 2+ P 3 Na 1+ Cl 1 - Metals: The charge is always positive; number value is equal to the number of valence electrons. Non-metals: The charge is always negative; number value is equal to: 8 – valence electrons.
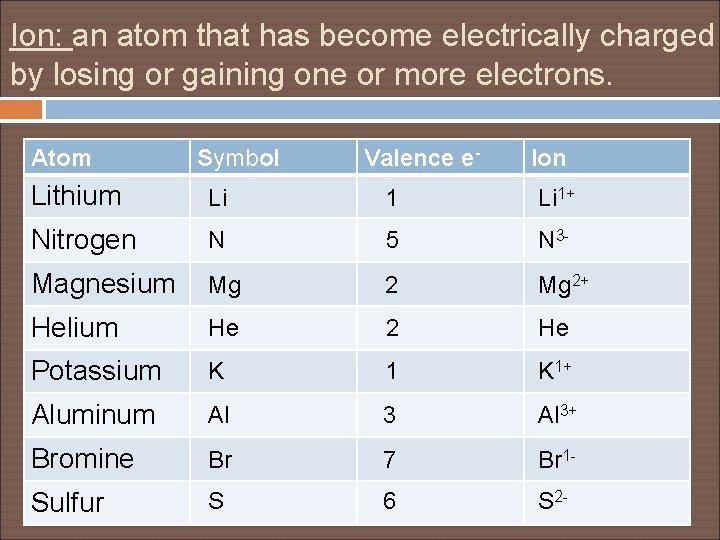
Ion: an atom that has become electrically charged by losing or gaining one or more electrons. Atom Symbol Valence e- Ion Lithium Li 1+ Nitrogen N 5 N 3 - Magnesium Mg 2+ Helium He 2 He Potassium K 1+ Aluminum Al 3+ Bromine Br 7 Br 1 - Sulfur S 6 S 2 -
RECAP! https: //www. youtube. com/watch? v=h. SNwds-H 1_0
- Slides: 11