Bonding Valence Electrons The electrons on the outside

Bonding

Valence Electrons • The electrons on the outside edge of the atom • This is where the action is- where bonding takes place • Atoms have no more than 8 valence electrons Neon 1 s 22 p 6 Argon 1 s 22 p 6 3 s 23 p 6 Radon [Xe]6 s 24 f 145 d 106 p 6
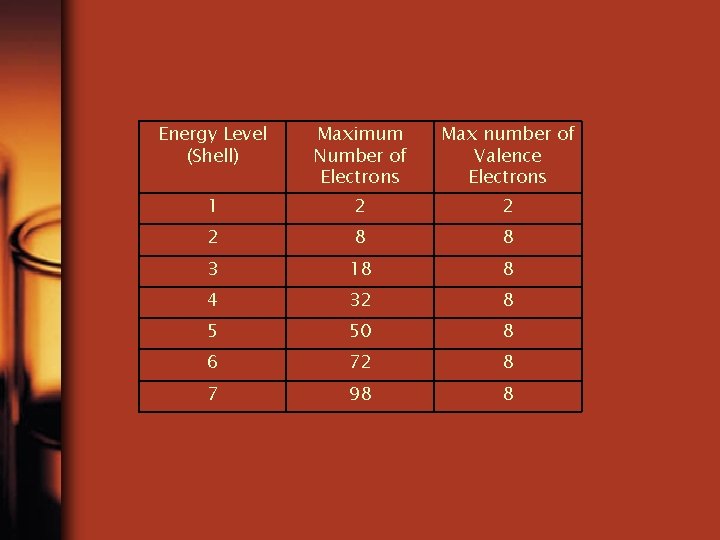
Energy Level (Shell) Maximum Number of Electrons Max number of Valence Electrons 1 2 2 2 8 8 3 18 8 4 32 8 5 50 8 6 72 8 7 98 8
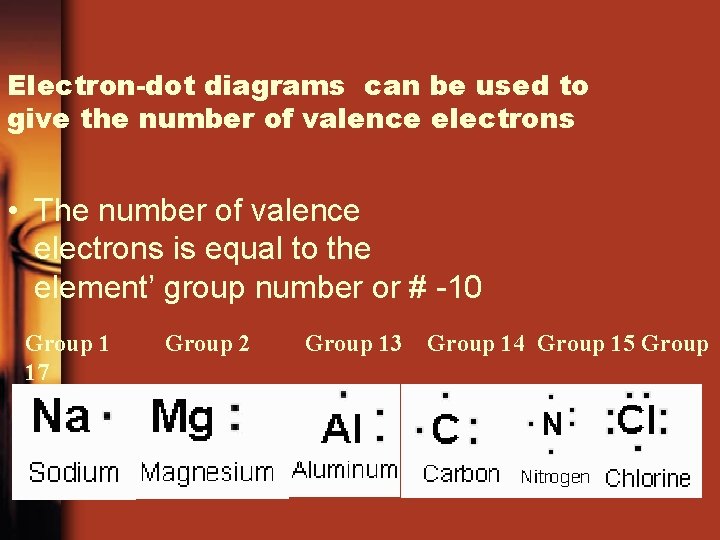
Electron-dot diagrams can be used to give the number of valence electrons • The number of valence electrons is equal to the element’ group number or # -10 Group 1 17 Group 2 Group 13 Group 14 Group 15 Group

• Write the electron-dot symbols for the following elements: iodine phosphorus gallium argon

The Octet Rule: • Atoms will combine to form compounds in order to reach eight electrons in their outer energy level. • Atoms with less than 4 electrons tend to lose electrons. • Atoms with more than 4 electrons tend to gain electrons. • Some atoms share electrons

Types of Chemical Bonds • Ionic bond - a transfer of one or more electrons from one atom to another – Forms attractions due to the opposite charges of the atoms • Covalent bond - attractive force due to the sharing of electrons between atoms • Some bonds have characteristics of both types and not easily identified as one or the other

Why do compounds form? • Atoms are trying to get 8 valence electrons How do compounds form? • By ionic or covalent bonding How can you tell if a compound is ionic or covalent? • By the types of elements in the compound
• Ionic compounds form between metals and nonmetals
• Covalent compounds form between 2 nonmetals

Note Question 4: Indicate whether a bond between the following would be 1) Ionic 2) covalent ____ A. sodium and oxygen ____ B. nitrogen and oxygen ____ C. phosphorus and chlorine ____ D. calcium and sulfur ____ E. chlorine and bromine

Ions • Atoms with extra electrons or missing electrons – Extra electrons give an ion a negative charge – Missing electrons give an ion a positive charge If they have to choose, atoms would rather be stable than neutral.

How Does This Happen? Some atoms have a few too many electrons Some atoms only need a few electrons

What do you do if you are a sodium (Na) atom? Go look for an atom that wants the extra electron!
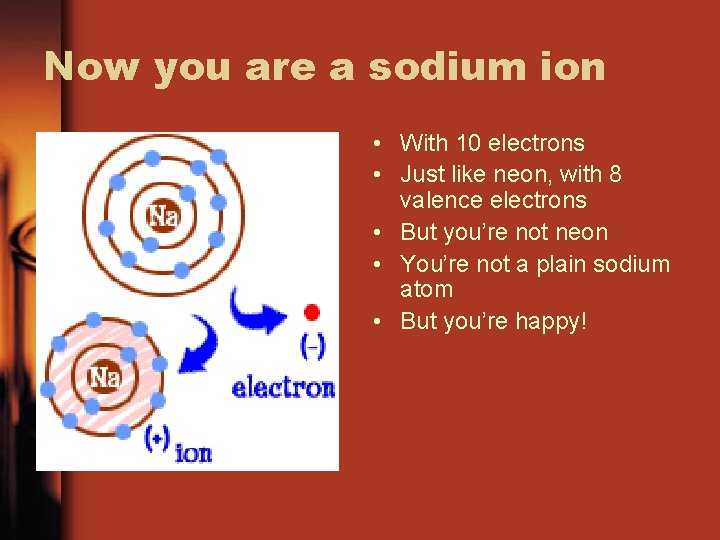
Now you are a sodium ion • With 10 electrons • Just like neon, with 8 valence electrons • But you’re not neon • You’re not a plain sodium atom • But you’re happy!
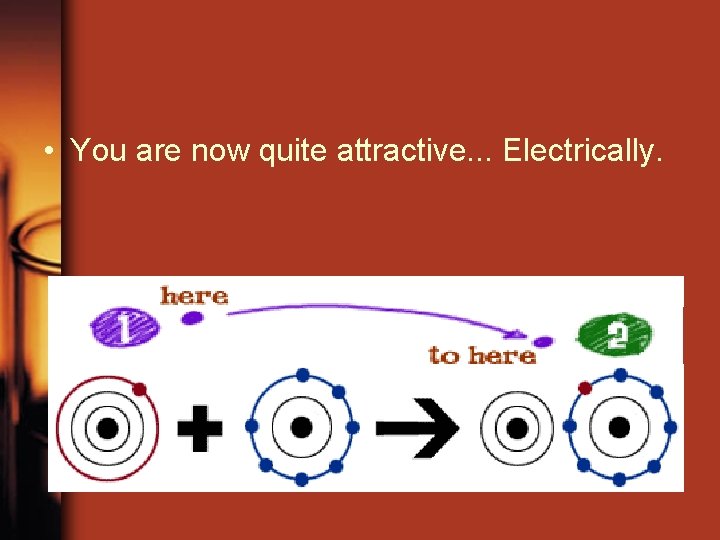
• You are now quite attractive. . . Electrically.
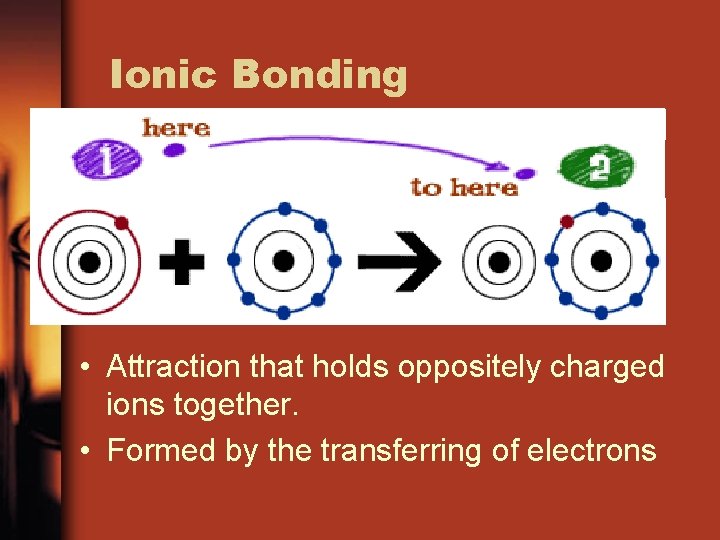
Ionic Bonding • Attraction that holds oppositely charged ions together. • Formed by the transferring of electrons

Ions from Metal Ions n In ionic compounds, metals in 1, 2, 13 and 3 -12 lose electrons to nonmetals n Metals lose electrons to achieve the octet arrangement in the next lowest energy level n The names of metal ions are the same as the elements n Metal ionic charge: +1, +2, +3, or +4
Ions from Nonmetals n. In ionic compounds, nonmetals in 15, 16, and 17 gain electrons from metals n. Nonmetal add electrons to achieve the octet arrangement n. Nonmetal ionic charge: -3, -2, or -1 n. The names of nonmetal ions end in -ide
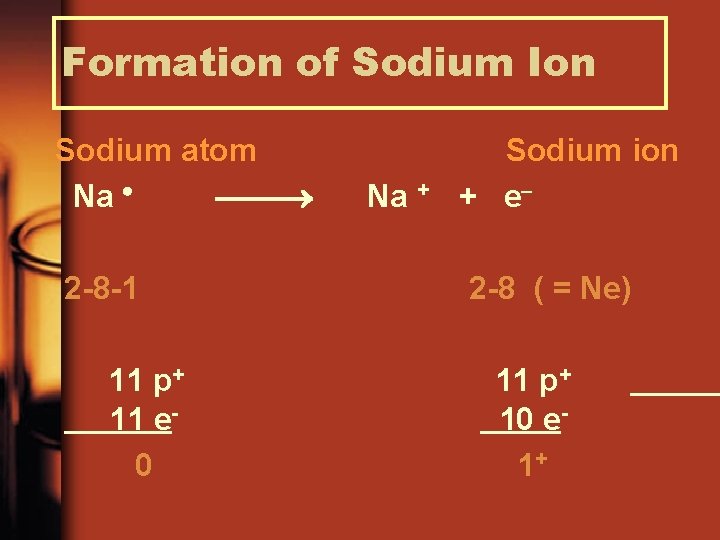
Formation of Sodium Ion Sodium atom Na 2 -8 -1 11 p+ 11 e 0 Na + Sodium ion + e 2 -8 ( = Ne) 11 p+ 10 e 1+
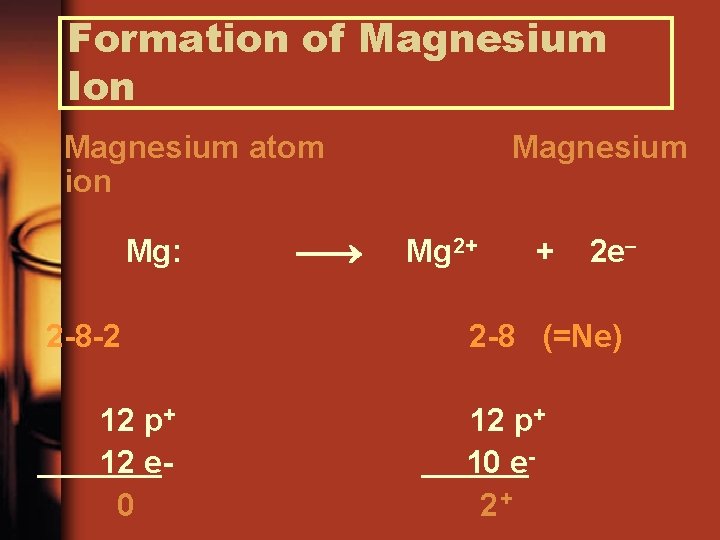
Formation of Magnesium Ion Magnesium atom ion Mg: 2 -8 -2 12 p+ 12 e 0 Magnesium Mg 2+ + 2 e 2 -8 (=Ne) 12 p+ 10 e 2+
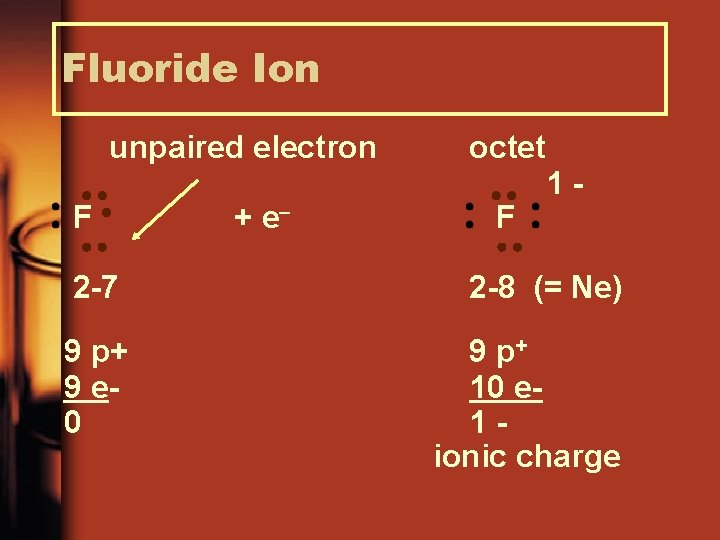
Fluoride Ion unpaired electron F 2 -7 9 p+ 9 e 0 + e octet F 1 - 2 -8 (= Ne) 9 p+ 10 e 1 ionic charge
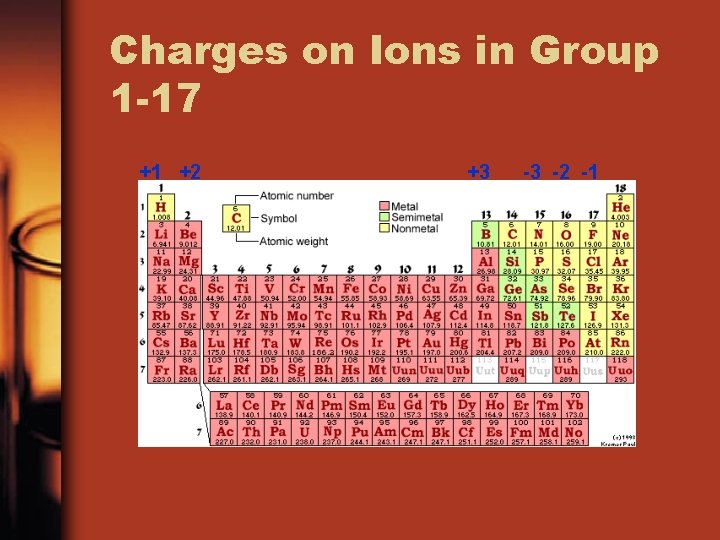
Charges on Ions in Group 1 -17 +1 +2 +3 -3 -2 -1

Ions A. Number of valence electrons in aluminum 1) 1 e 2) 2 e 3) 3 e. B. Change in electrons for octet 1) lose 3 e 2) gain 3 e- 3) gain 5 e- C. Ionic charge of aluminum 1) 32) 5 - 3) 3+

Learning Check B 3 Give the ionic charge for each of the following: A. 12 p+ and 10 e 1) 0 2) 2+ 3) 2 B. 50 p+ and 46 e 1) 2+ 2) 4+ 3) 4 - C. 15 p+ and 18 e 2) 3+ 2) 3 - 3) 5 -
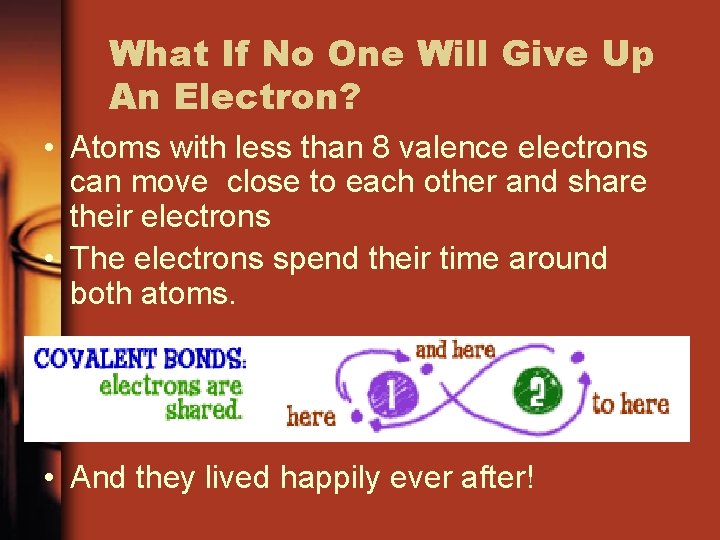
What If No One Will Give Up An Electron? • Atoms with less than 8 valence electrons can move close to each other and share their electrons • The electrons spend their time around both atoms. • And they lived happily ever after!
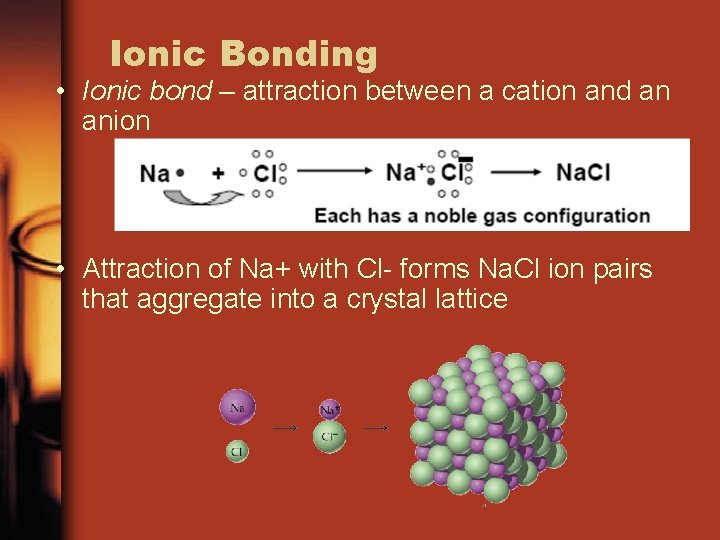
Ionic Bonding • Ionic bond – attraction between a cation and an anion • Attraction of Na+ with Cl- forms Na. Cl ion pairs that aggregate into a crystal lattice

Features of Ionic Bonding • Ion formation takes place by electron transfer • The ions are held together by the electrostatic force of the opposite charges • Reactions between metals and nonmetals (representative elements tend to be ionic)

Ionic Compound Properties • brittle • high melting points • conduct electricity in molten state or when dissolved in water

Covalent Bonding • Let’s look at the formation of H 2: H + H H 2 • Each hydrogen has one electron in its valance shell • Both hydrogen atoms have an equal tendency to gain or lose electrons • Electron transfer from one H to another usually will not occur under normal conditions (No one will let go!)
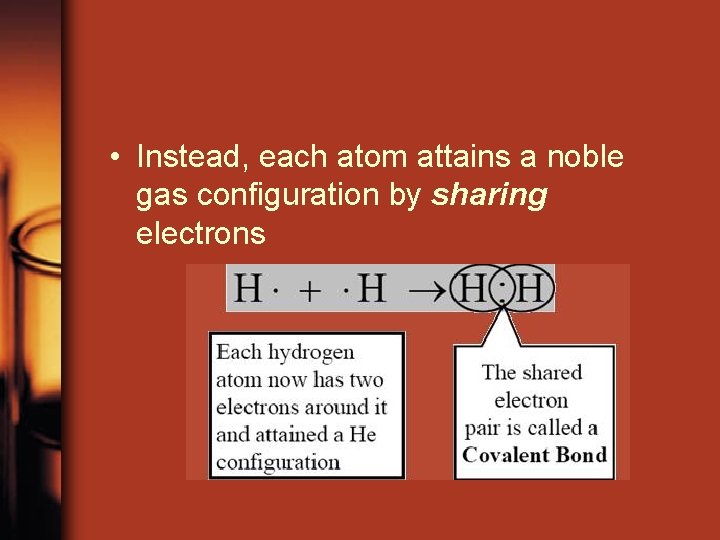
• Instead, each atom attains a noble gas configuration by sharing electrons
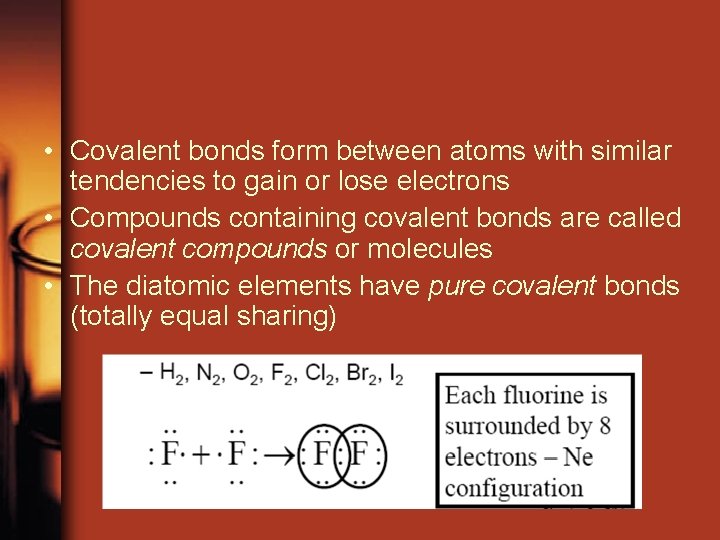
• Covalent bonds form between atoms with similar tendencies to gain or lose electrons • Compounds containing covalent bonds are called covalent compounds or molecules • The diatomic elements have pure covalent bonds (totally equal sharing)

• The Polar Covalent Bond – Ionic bonding involves the transfer of electrons – Covalent bonding involves the sharing of electrons • Polar covalent bonding - bonds made up of unequally shared electron pairs
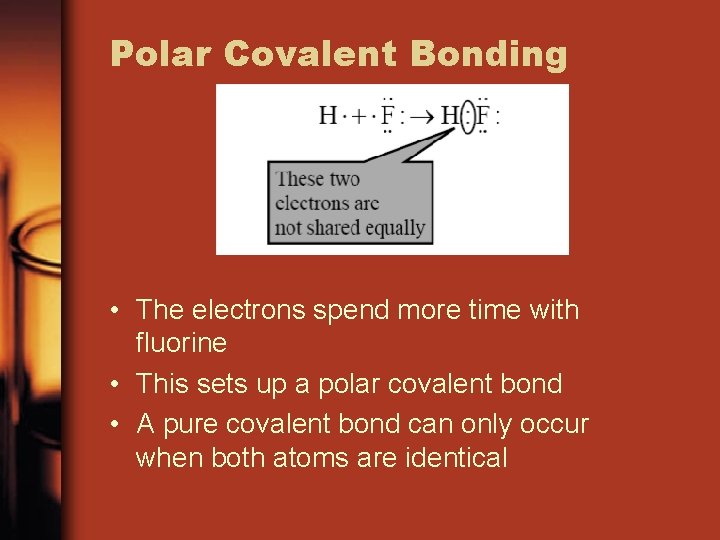
Polar Covalent Bonding • The electrons spend more time with fluorine • This sets up a polar covalent bond • A pure covalent bond can only occur when both atoms are identical

Electronegativity • Electronegativity - a measure of the ability of an atom to attract electrons in a chemical bond • Elements with high electronegativity have a greater ability to attract electrons than do elements with low electronegativity • The difference in electronegativity determines the extent of bond polarity
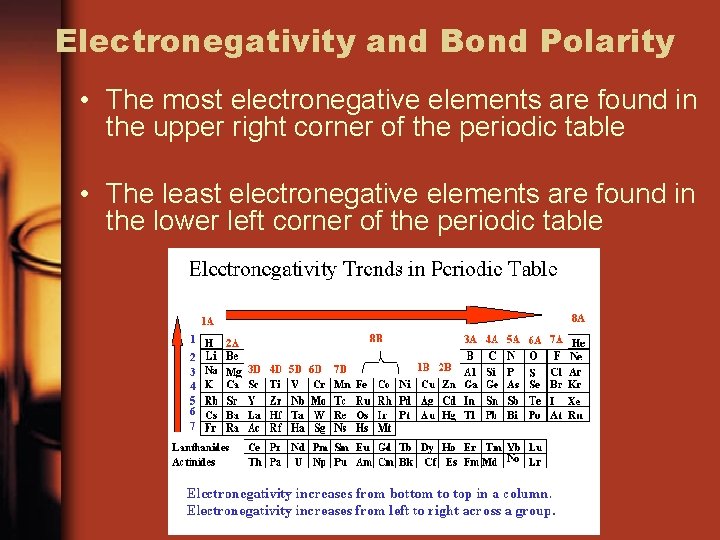
Electronegativity and Bond Polarity • The most electronegative elements are found in the upper right corner of the periodic table • The least electronegative elements are found in the lower left corner of the periodic table

Know the trend! • Which is more electronegative • Boron or gallium? • Calcium or zinc?
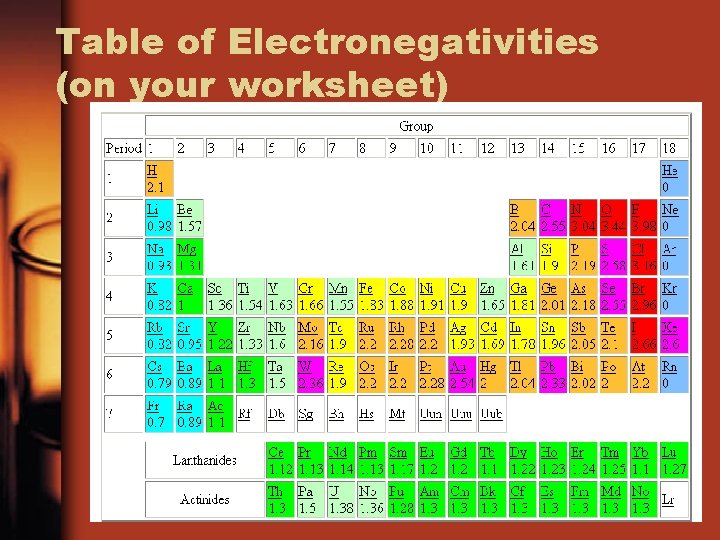
Table of Electronegativities (on your worksheet)
Electronegativity and Bond Polarity • The greater the difference in electronegativity between two atoms, the greater the polarity of their bond • Which would be more polar, a H-F bond or H-Cl bond? • H-F … 4. 0 - 2. 1 = 1. 9 • H-Cl … 3. 0 - 2. 1 = 0. 9 • The HF bond is more polar than the HCl bond

Look at your handout • There is a chart in the lower right hand corner of the back • This gives the break-off points for deciding what type of bond you have between two atoms • If the difference is 0, Pure covalent • If the difference is ≤ 0. 4, Nonpolar covalent • If the difference is 0. 5 -1. 7, Polar covalent • If the difference is >1. 7, Ionic

• Using the table of electronegativites, classify the following bonds as ionic, polar covalent or nonpolar covalent. • BCl 3 • CS 2

• There is no sharp distinction between bonding types. • The positive end (or pole) in a polar bond is represented δ+ and the negative pole δ-.

Dipole Moments • Consider HF: • – The difference in electronegativity leads to a polar bond. • – There is more electron density on F than on H. • – Since there are two different “ends” of the molecule, we • call HF a dipole.
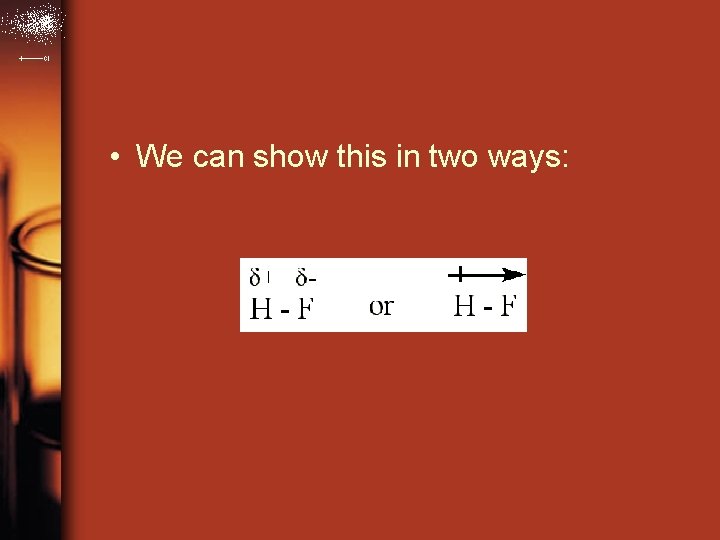
• We can show this in two ways:
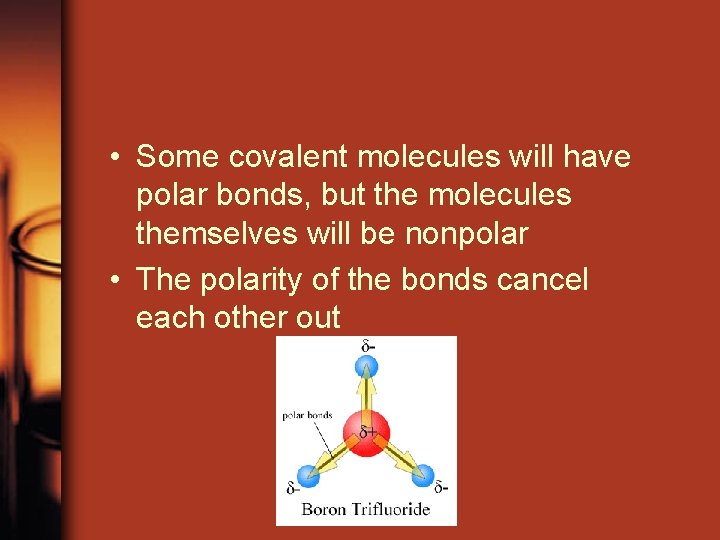
• Some covalent molecules will have polar bonds, but the molecules themselves will be nonpolar • The polarity of the bonds cancel each other out
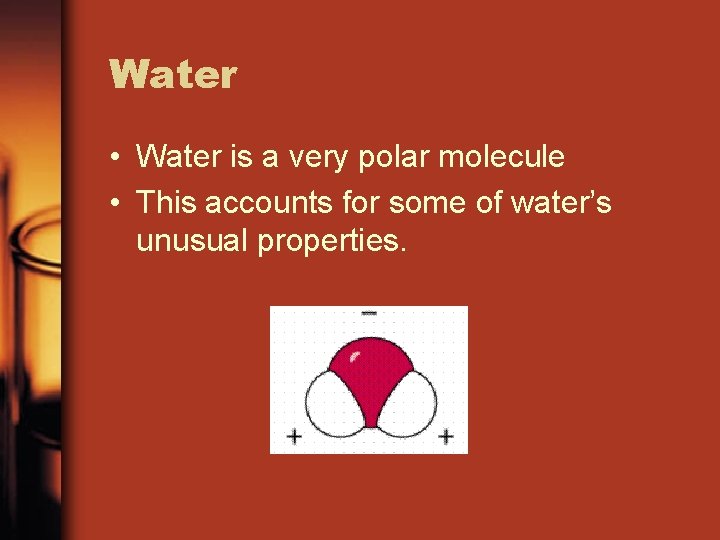
Water • Water is a very polar molecule • This accounts for some of water’s unusual properties.

• Water dissolves ionic and polar substances
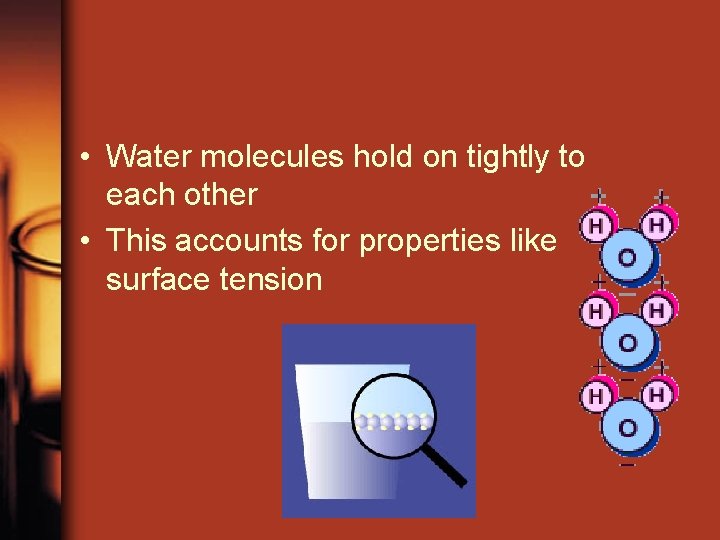
• Water molecules hold on tightly to each other • This accounts for properties like surface tension

• Water has a high heat capacity • Water is a liquid at room temperature, even though other compounds in its size range are gases
- Slides: 49