Bonding the Periodic Table 1 Atoms Bonding and
Bonding & the Periodic Table 1
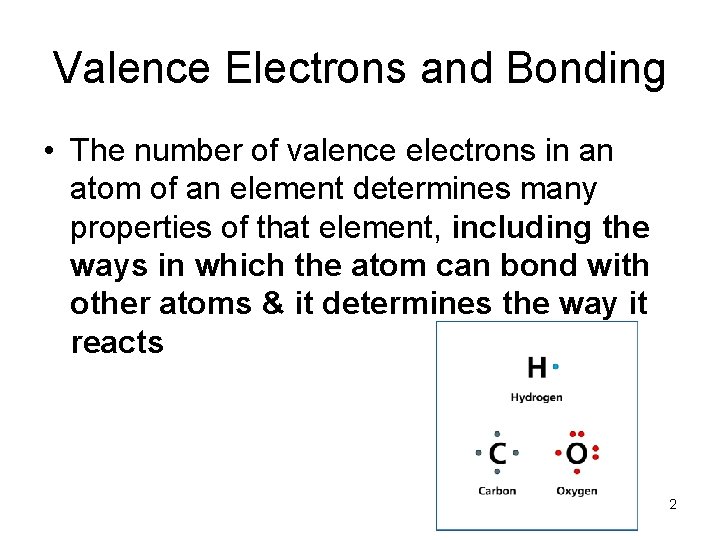
- Atoms, Bonding, and the Periodic Table Valence Electrons and Bonding • The number of valence electrons in an atom of an element determines many properties of that element, including the ways in which the atom can bond with other atoms & it determines the way it reacts 2
Fundamental Rule • All atoms want to look like the noble gasses – So they either want to get rid of all of their valence electrons, or accept more electrons. 3
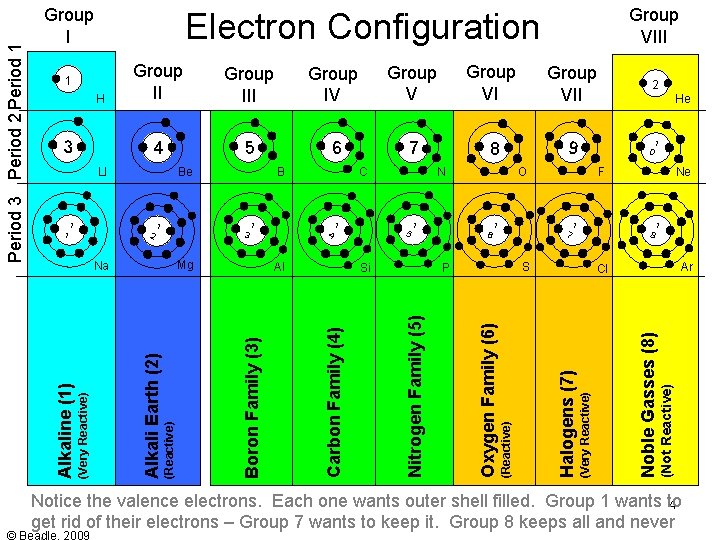
Group III Group IV Group VII 2 4 5 6 7 8 9 1 0 Si F 1 7 1 8 S P Ne Ar Cl (Not Reactive) Al 1 6 He Noble Gasses (8) (Reactive) Alkali Earth (2) 1 5 1 4 O (Very Reactive) Mg Na N Halogens (7) 1 3 1 2 C (Reactive) B Oxygen Family (6) 1 1 Be Nitrogen Family (5) LI Carbon Family (4) 3 Group II Boron Family (3) H (Very Reactive) Group VIII Electron Configuration 1 Alkaline (1) Period 3 Period 2 Period 1 Group I Notice the valence electrons. Each one wants outer shell filled. Group 1 wants to 4 get rid of their electrons – Group 7 wants to keep it. Group 8 keeps all and never
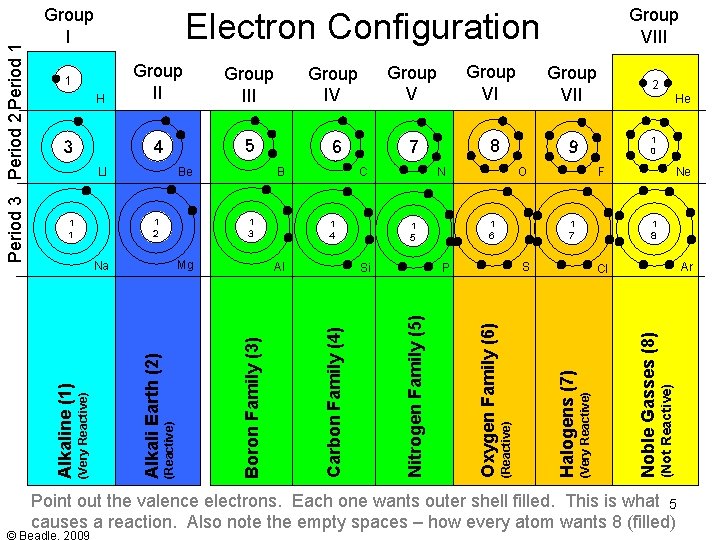
Group III Group IV Group VII 2 4 5 6 7 8 9 1 0 1 8 S P Ne Ar Cl (Not Reactive) Si F 1 7 1 6 1 5 He Noble Gasses (8) (Reactive) Alkali Earth (2) Al O (Very Reactive) Mg Na 1 4 N Halogens (7) 1 3 C (Reactive) 1 2 1 1 B Oxygen Family (6) Be Nitrogen Family (5) LI Carbon Family (4) 3 Group II Boron Family (3) H (Very Reactive) Group VIII Electron Configuration 1 Alkaline (1) Period 3 Period 2 Period 1 Group I Point out the valence electrons. Each one wants outer shell filled. This is what 5 causes a reaction. Also note the empty spaces – how every atom wants 8 (filled)
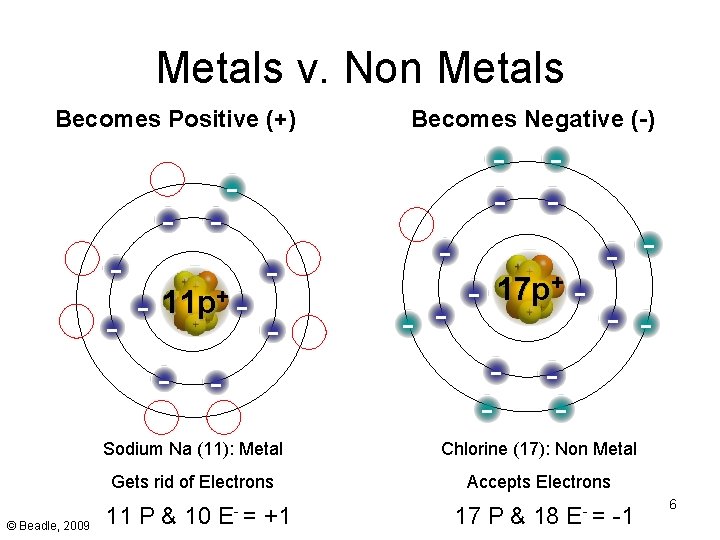
Metals v. Non Metals Becomes Positive (+) - - 11 p+ - © Beadle, 2009 - Becomes Negative (-) - - - + 17 p - - - Sodium Na (11): Metal Chlorine (17): Non Metal Gets rid of Electrons Accepts Electrons 11 P & 10 E- = +1 17 P & 18 E- = -1 6
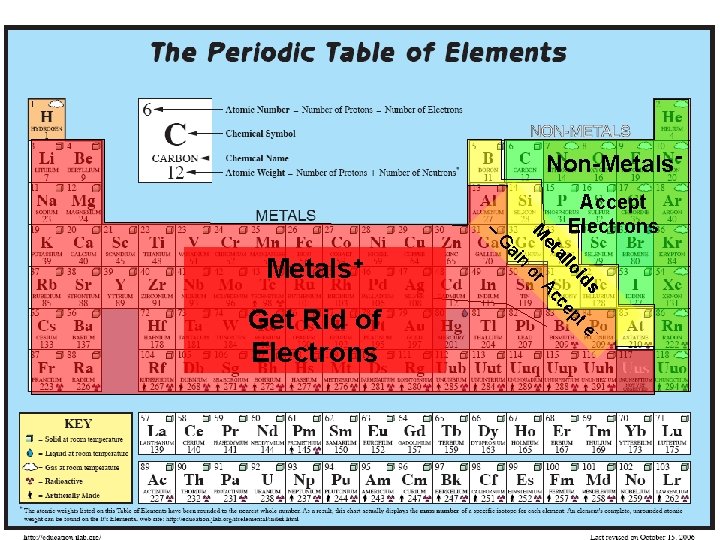
Non-Metals- A or s te id lo cep c al et n ai G Get Rid of Electrons M – Metals+ Accept Electrons 7
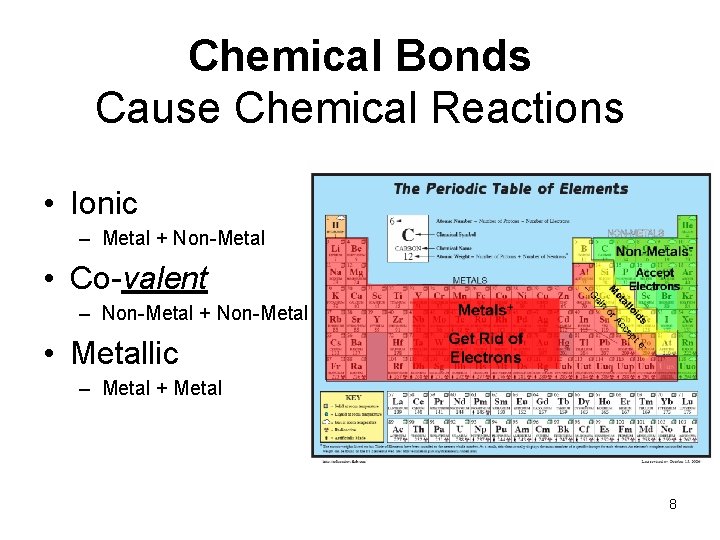
Chemical Bonds Cause Chemical Reactions • Ionic – Metal + Non-Metal • Co-valent – Non-Metal + Non-Metal • Metallic – Metal + Metal 8
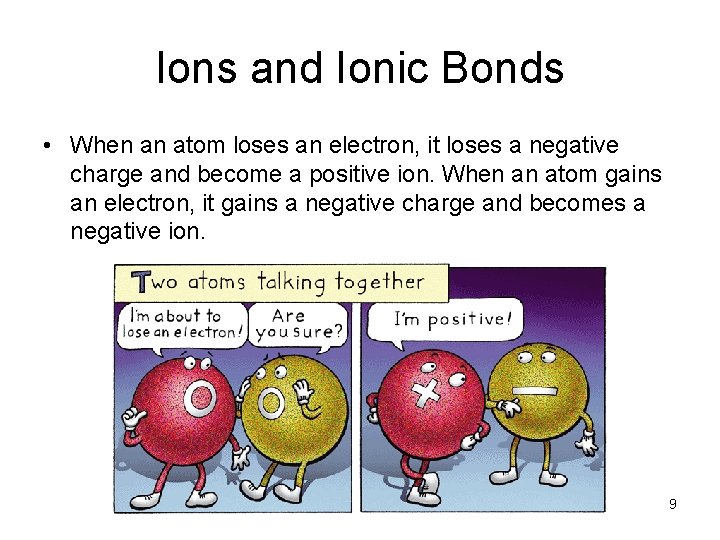
- Ionic Bonds Ions and Ionic Bonds • When an atom loses an electron, it loses a negative charge and become a positive ion. When an atom gains an electron, it gains a negative charge and becomes a negative ion. 9
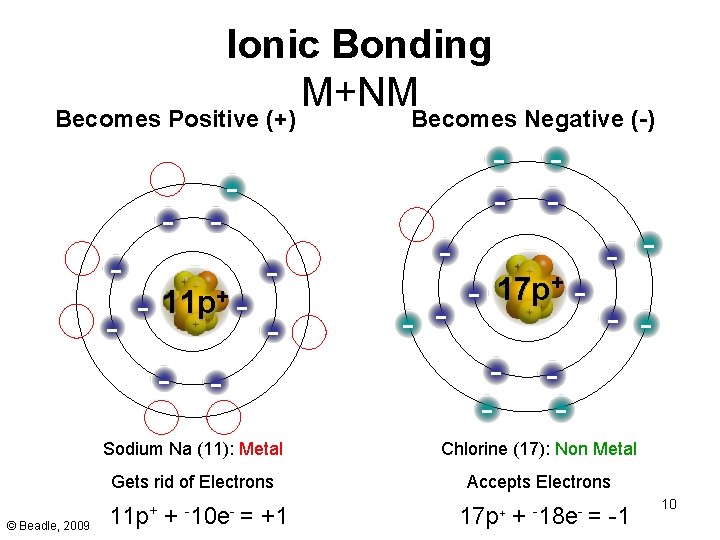
Ionic Bonding M+NM Becomes Positive (+) - - 11 p+ - © Beadle, 2009 - Becomes Negative (-) - - - + 17 p - - - Sodium Na (11): Metal Chlorine (17): Non Metal Gets rid of Electrons Accepts Electrons 11 p+ + -10 e- = +1 17 p+ + -18 e- = -1 10
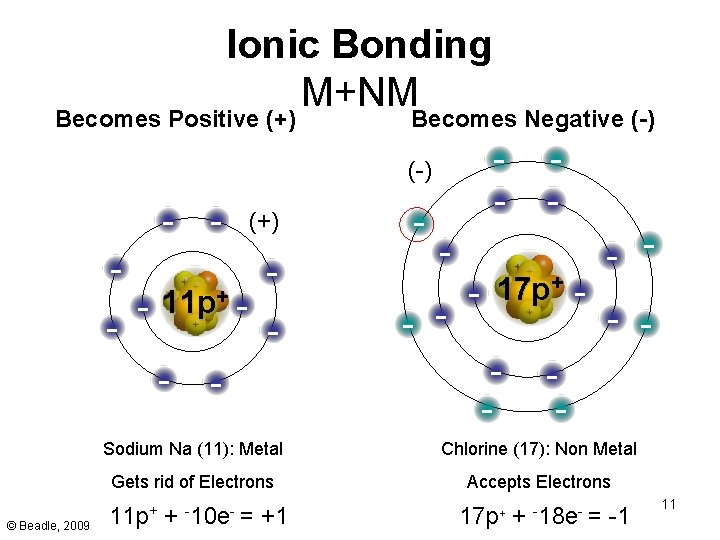
Ionic Bonding M+NM Becomes Positive (+) Becomes Negative (-) - - 11 p+ - © Beadle, 2009 - (+) - - - - + 17 p - - - Sodium Na (11): Metal Chlorine (17): Non Metal Gets rid of Electrons Accepts Electrons 11 p+ + -10 e- = +1 17 p+ + -18 e- = -1 11

Example: Salt Crystal 13
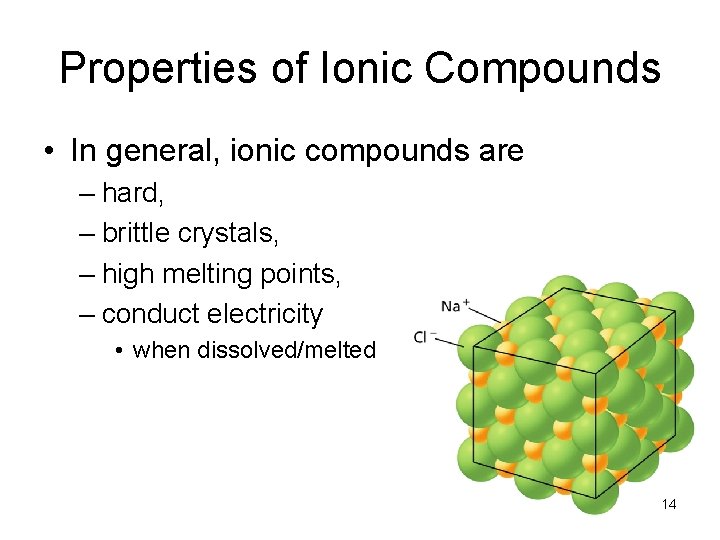
- Ionic Bonds Properties of Ionic Compounds • In general, ionic compounds are – hard, – brittle crystals, – high melting points, – conduct electricity • when dissolved/melted 14
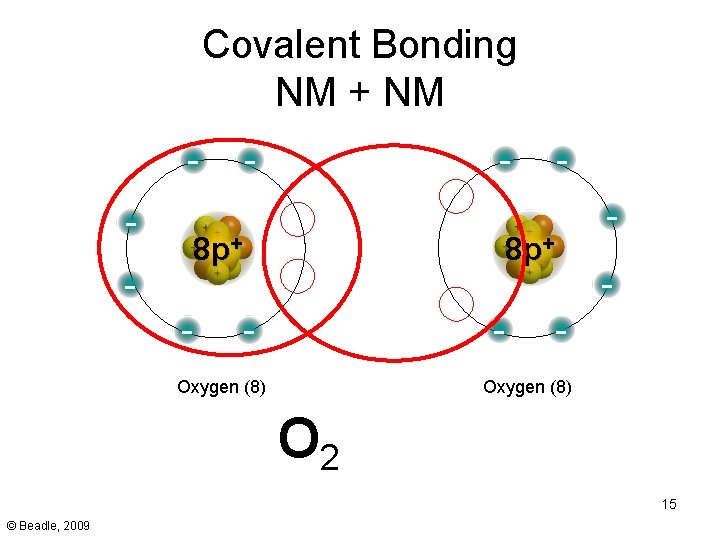
Covalent Bonding NM + NM - - - 8 p+ - - Oxygen (8) O 2 15 © Beadle, 2009
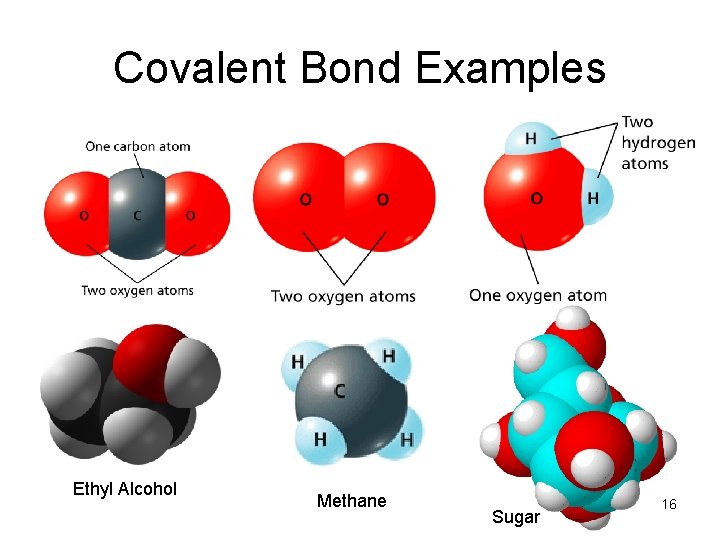
Covalent Bond Examples Ethyl Alcohol Methane Sugar 16
Properties of Covalent Bonds • Covalently Bonded Molecules: – Usually liquid/gas at room temp. • Sometimes you may find it in crystal form – Have low melting/boiling points – Have low viscosity – Have low density – Do not conduct electricity when dissolved. 17
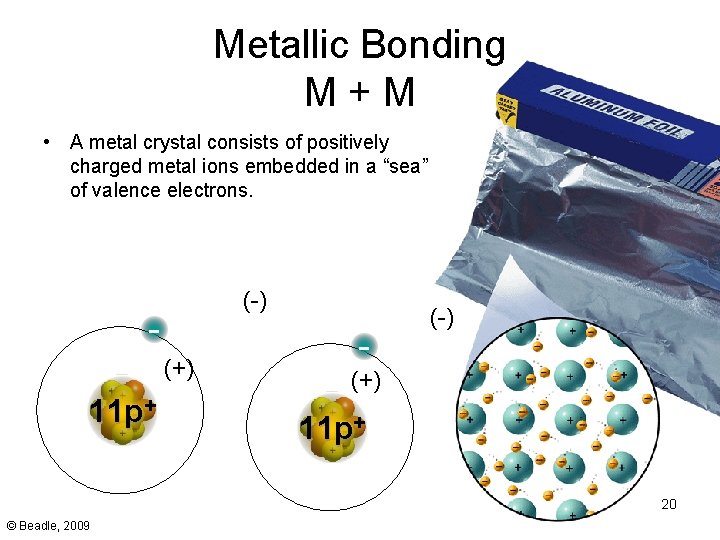
- Bonding in Metals Metallic Bonding M+M • A metal crystal consists of positively charged metal ions embedded in a “sea” of valence electrons. (-) (+) 11 p+ - (-) (+) 11 p+ 20 © Beadle, 2009
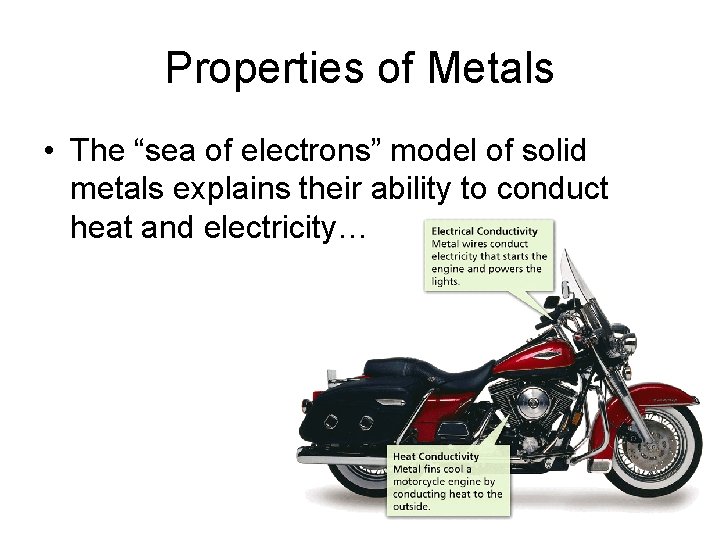
Properties of Metals • The “sea of electrons” model of solid metals explains their ability to conduct heat and electricity… 21
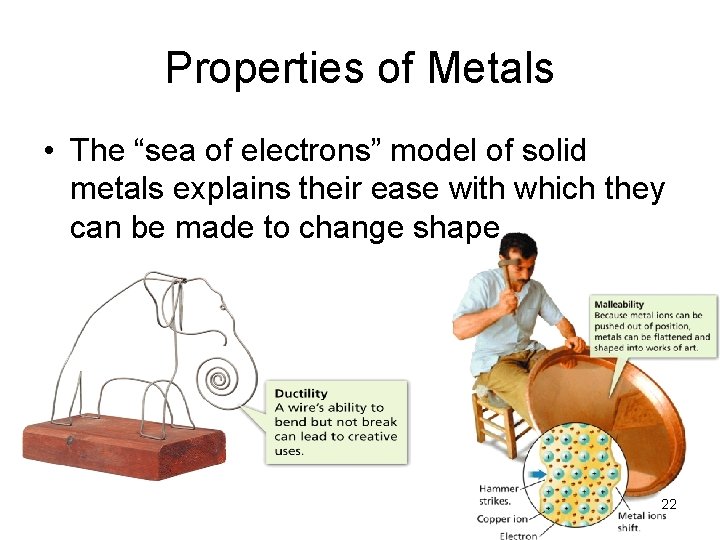
Properties of Metals • The “sea of electrons” model of solid metals explains their ease with which they can be made to change shape… 22
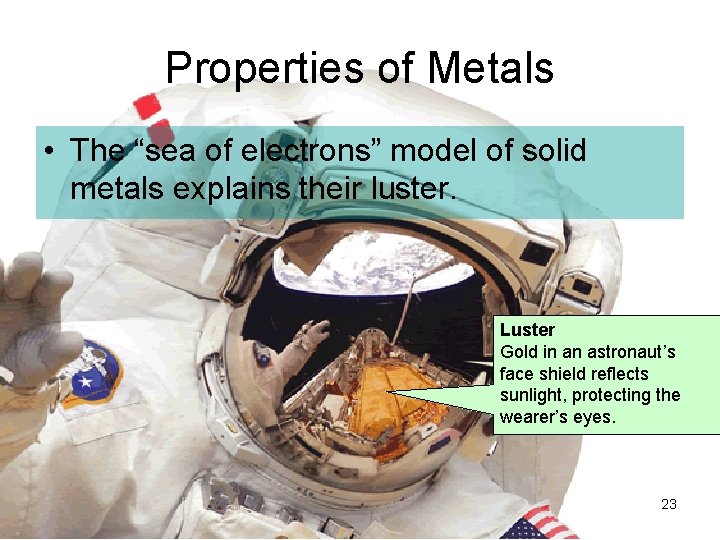
Properties of Metals • The “sea of electrons” model of solid metals explains their luster. Luster Gold in an astronaut’s face shield reflects sunlight, protecting the wearer’s eyes. 23

Chemical Reactions • Chemical Reactions happen because of bonding. 24
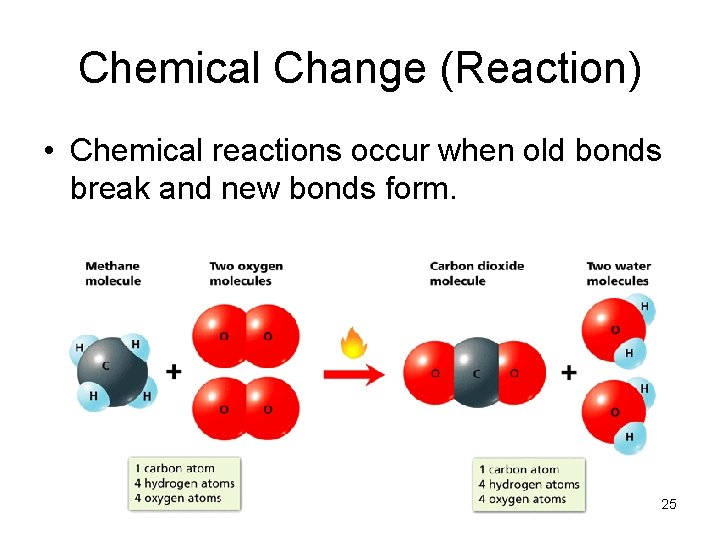
Chemical Change (Reaction) • Chemical reactions occur when old bonds break and new bonds form. 25
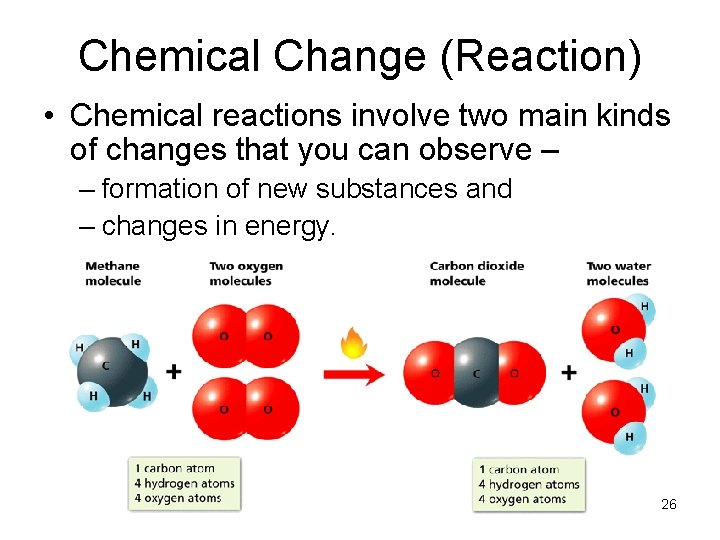
Chemical Change (Reaction) • Chemical reactions involve two main kinds of changes that you can observe – – formation of new substances and – changes in energy. 26
- Slides: 23