Bonding Science 9 Unit 5 Bonding Bonding is

Bonding Science 9: Unit 5
Bonding • Bonding is influence by the number of electrons in an atoms valence shell.
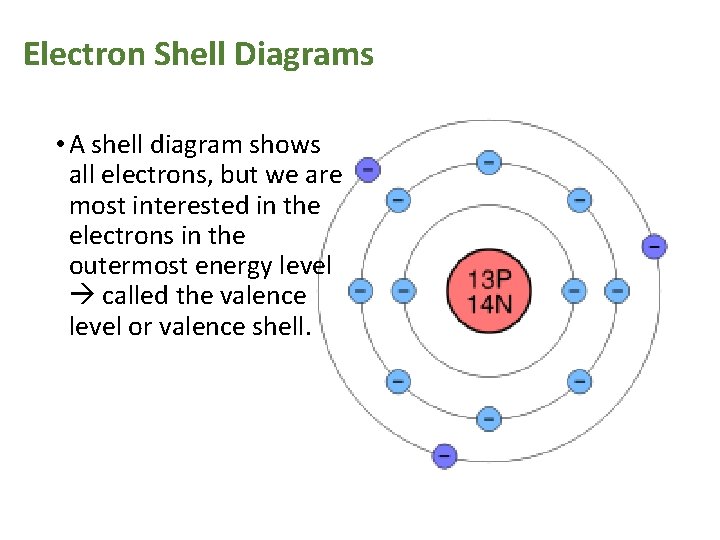
Electron Shell Diagrams • A shell diagram shows all electrons, but we are most interested in the electrons in the outermost energy level called the valence level or valence shell.
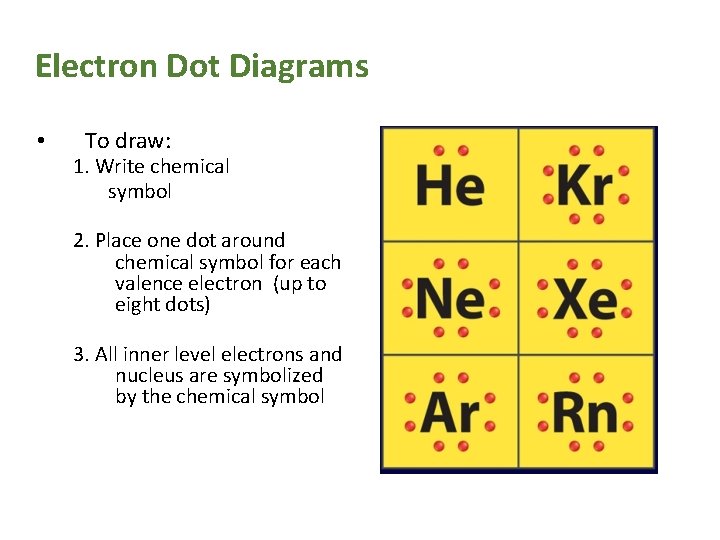
Electron Dot Diagrams • To draw: 1. Write chemical symbol 2. Place one dot around chemical symbol for each valence electron (up to eight dots) 3. All inner level electrons and nucleus are symbolized by the chemical symbol
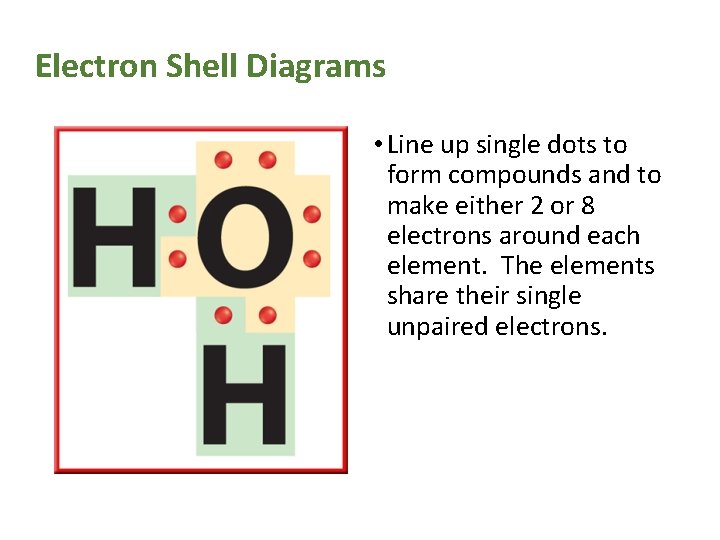
Electron Shell Diagrams • Line up single dots to form compounds and to make either 2 or 8 electrons around each element. The elements share their single unpaired electrons.
Compounds • A chemical formula is used to represent a compound • A chemical formula is a group of symbols that show the number and kind of atoms. • Examples • Na. Cl is made of one atom of Sodium (Na) and one atom of chlorine (Cl)

Octet Rule • In order for atom to be “happy” it must have a full valence shell (8). • Hydrogen, and Helium are exceptions since it will only have 2 in their outer level. • This full outer energy level is a very stable arrangement for atoms.
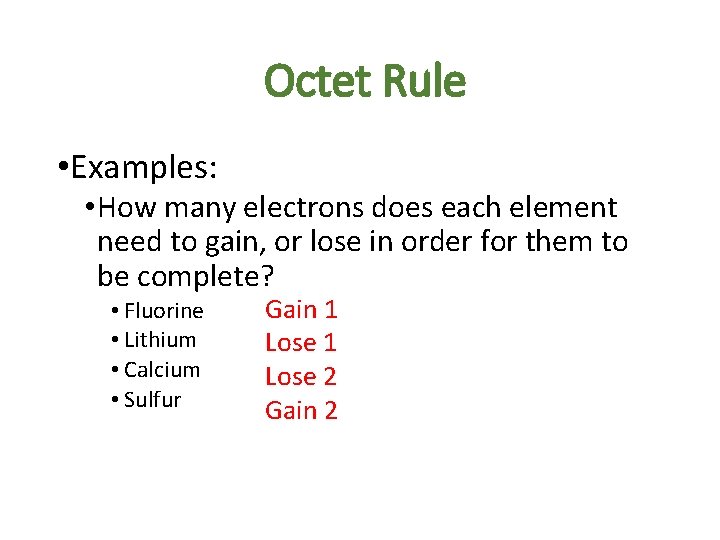
Octet Rule • Examples: • How many electrons does each element need to gain, or lose in order for them to be complete? • Fluorine • Lithium • Calcium • Sulfur Gain 1 Lose 2 Gain 2
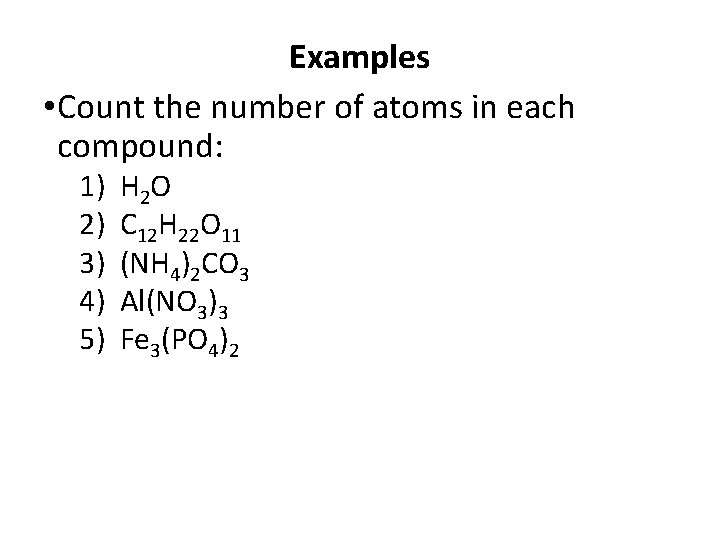
Examples • Count the number of atoms in each compound: 1) 2) 3) 4) 5) H 2 O C 12 H 22 O 11 (NH 4)2 CO 3 Al(NO 3)3 Fe 3(PO 4)2
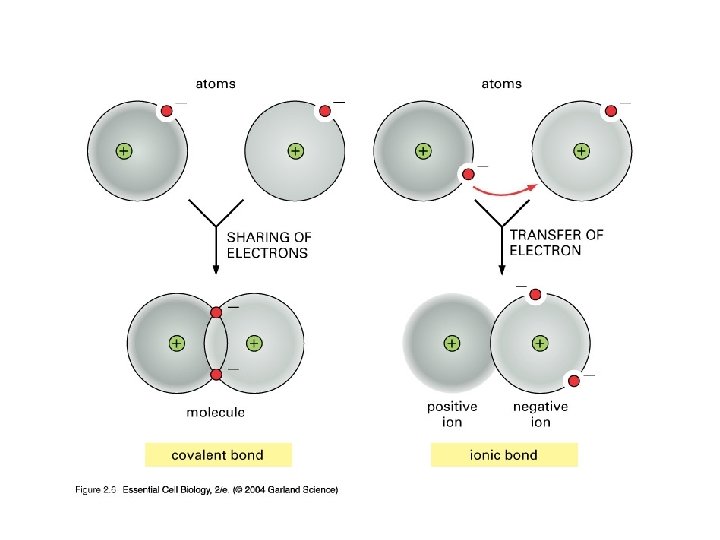
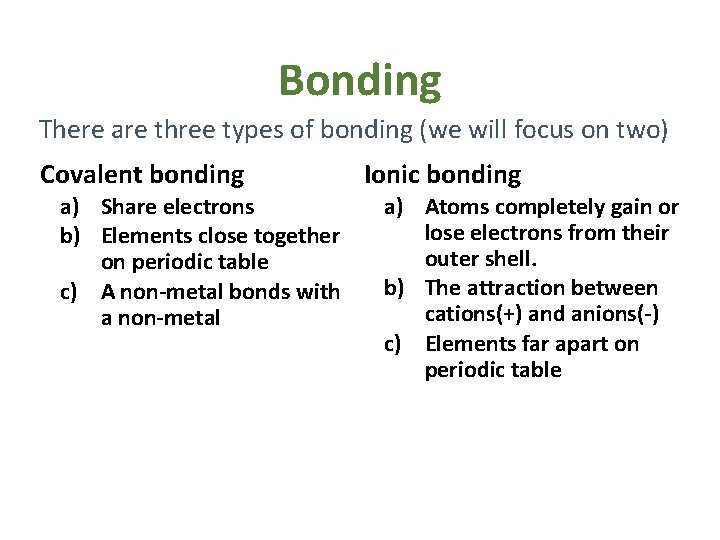
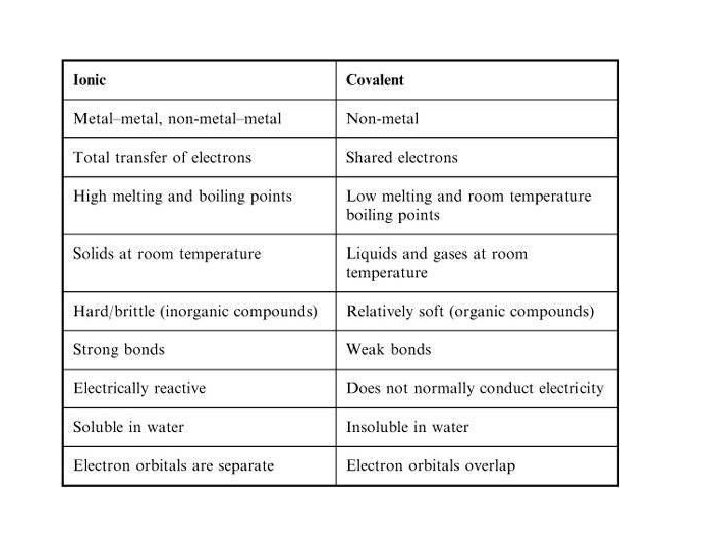
Bonding There are three types of bonding (we will focus on two) Covalent bonding a) Share electrons b) Elements close together on periodic table c) A non-metal bonds with a non-metal Ionic bonding a) Atoms completely gain or lose electrons from their outer shell. b) The attraction between cations(+) and anions(-) c) Elements far apart on periodic table
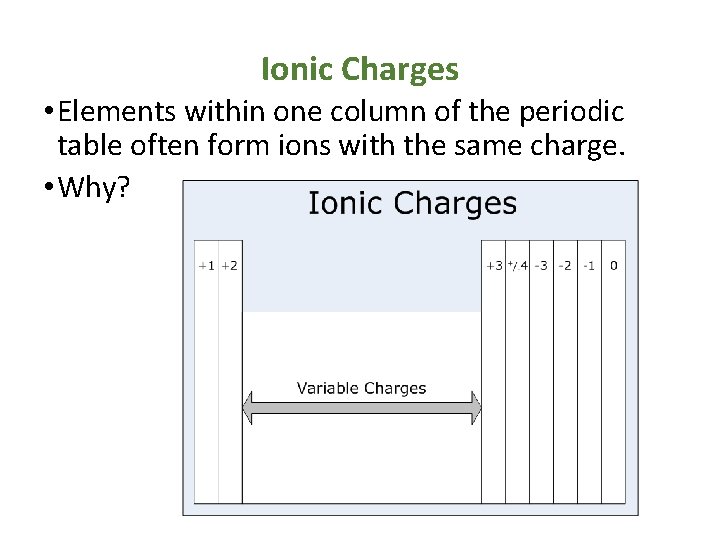
Ionic Charges • Elements within one column of the periodic table often form ions with the same charge. • Why?
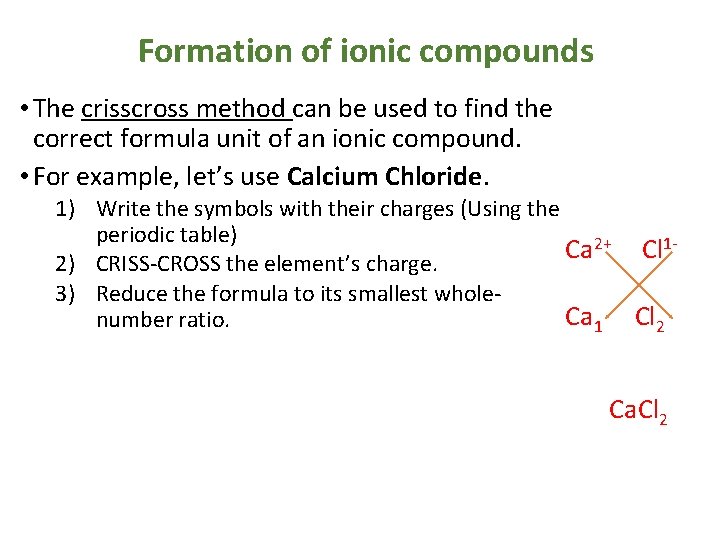
Formation of ionic compounds • The crisscross method can be used to find the correct formula unit of an ionic compound. • For example, let’s use Calcium Chloride. 1) Write the symbols with their charges (Using the periodic table) 2+ 1 Ca Cl 2) CRISS-CROSS the element’s charge. 3) Reduce the formula to its smallest whole. Ca 1 Cl 2 number ratio. Ca. Cl 2

Examples Write the formulas for the following: 1. 2. 3. 4. 5. 6. Magnesium Oxide Lithium Sulfide Aluminum Bromide Boron Oxide Barium Bromide Potassium Sulfide 7. 8. 9. Potassium Nitride Nickel (II) Chloride Chromium (III) Oxide 10. Iron (III) Bromide 11. Calcium sulfide 12. Copper (II) chloride
Covalent Bonding Covalent bonding a) b) c) Results in the sharing of electrons between two atoms The shared electrons are “owned” equally by the two bonded atoms A non-metal bonds with a non-metal

Ionic or Covalent? 1) 2) 3) 4) 5) 6) CO 2 C 6 H 12 O 6 Na. Cl H 2 O Ca 3 P 2 Ni. Cl 3
Balancing chemical equations Questions you should be able to answer: 1. What are the different parts of the chemical equation? 2. How can we tell if a chemical equation is balanced or not? 3. How does balancing equation relate to the law of conservation of mass?
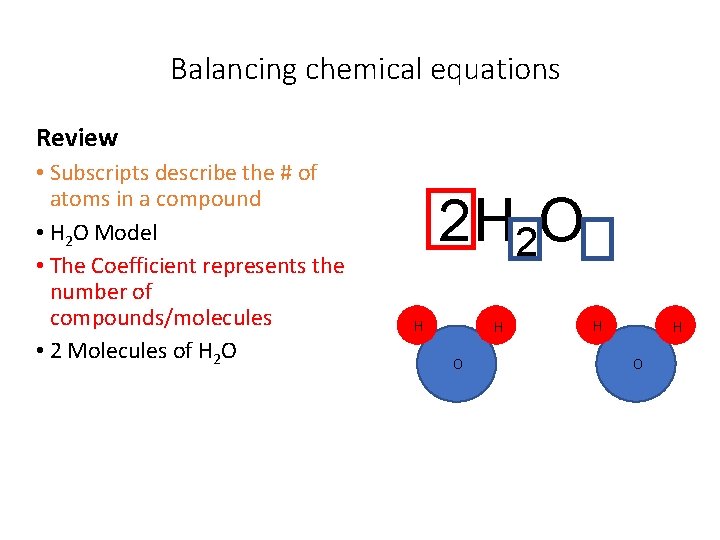
Balancing chemical equations Review • Subscripts describe the # of atoms in a compound • H 2 O Model • The Coefficient represents the number of compounds/molecules • 2 Molecules of H 2 O 2 H 2 O H H O
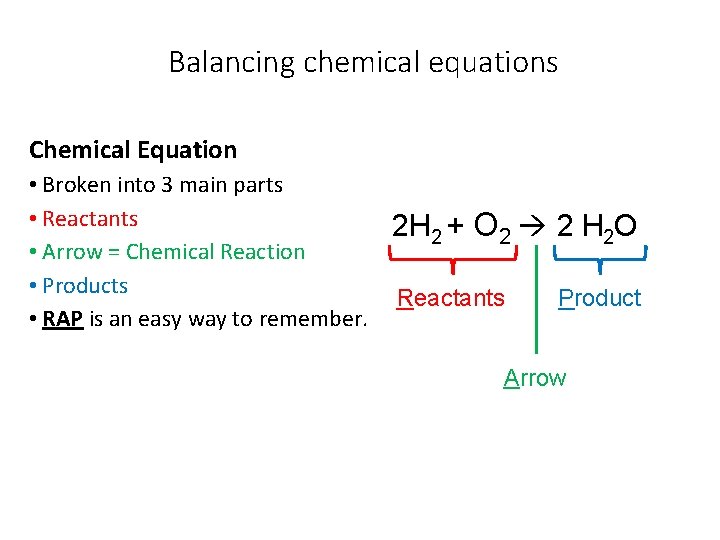
Balancing chemical equations Chemical Equation • Broken into 3 main parts • Reactants • Arrow = Chemical Reaction • Products • RAP is an easy way to remember. 2 H 2 + O 2 2 H 2 O Reactants Product Arrow
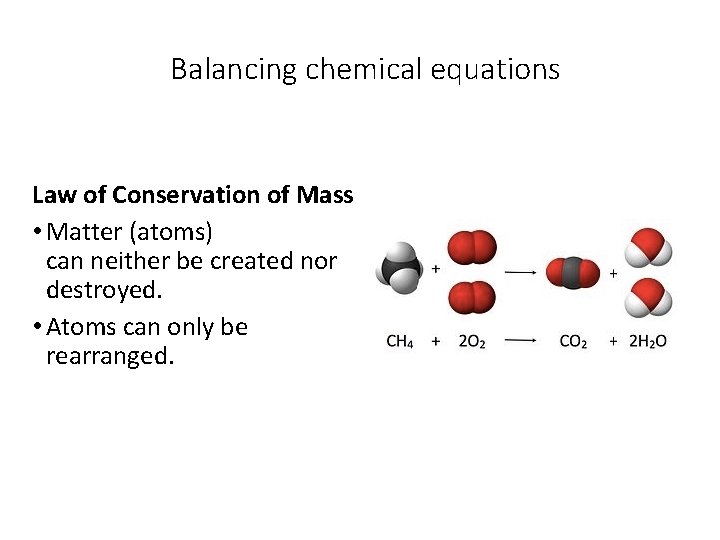
Balancing chemical equations Law of Conservation of Mass • Matter (atoms) can neither be created nor destroyed. • Atoms can only be rearranged.
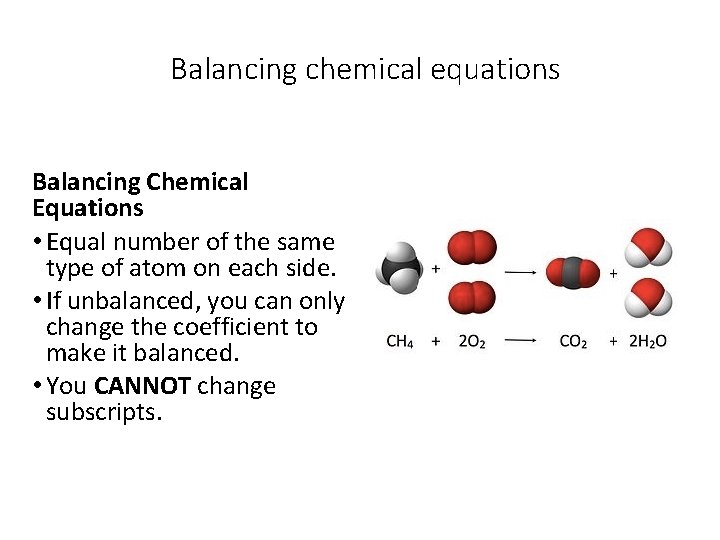
Balancing chemical equations Balancing Chemical Equations • Equal number of the same type of atom on each side. • If unbalanced, you can only change the coefficient to make it balanced. • You CANNOT change subscripts.
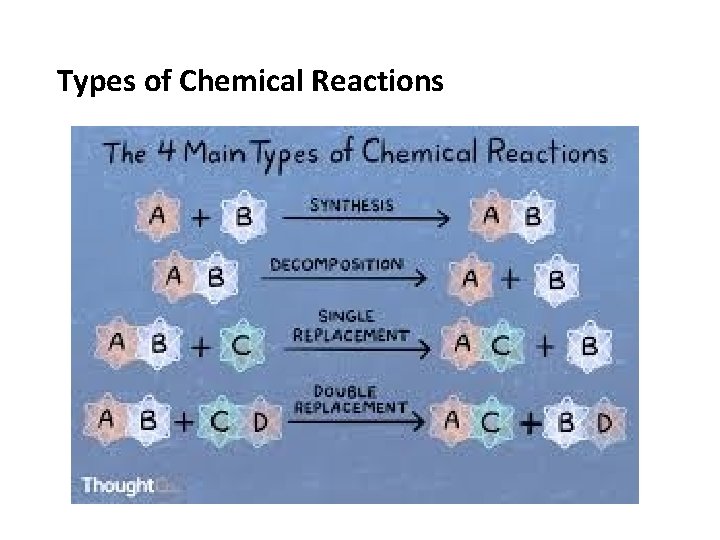
Types of Chemical Reactions
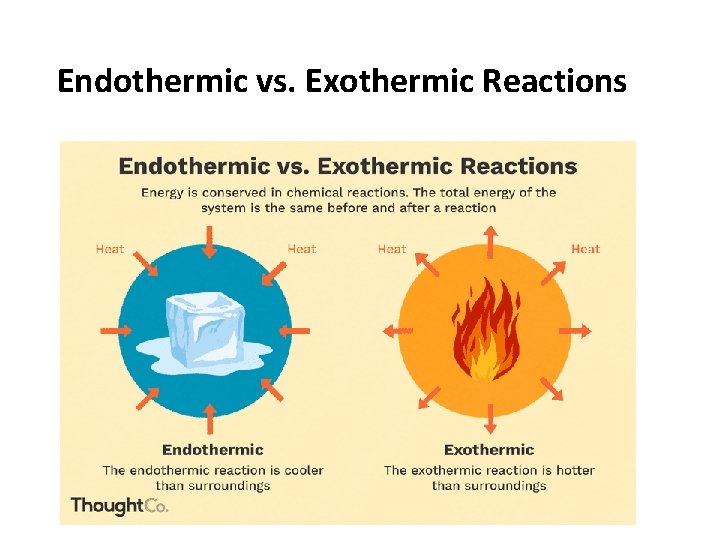
Endothermic vs. Exothermic Reactions
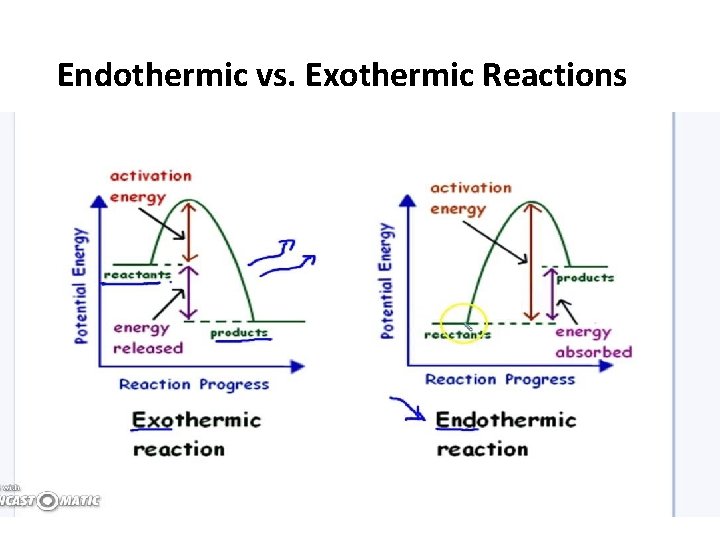
Endothermic vs. Exothermic Reactions

- Slides: 26