BONDING Molecules and Covalent Bonding Part 1 Molecules

BONDING Molecules and Covalent Bonding Part 1 Molecules and Covalent Bonding Formal Charge

Molecules A molecule is a discrete chemical entity, in which atoms are held together by the electrostatic attractions of covalent bonds. Electron sharing enables each atom in a covalent bond to acquire a noble gas configuration.
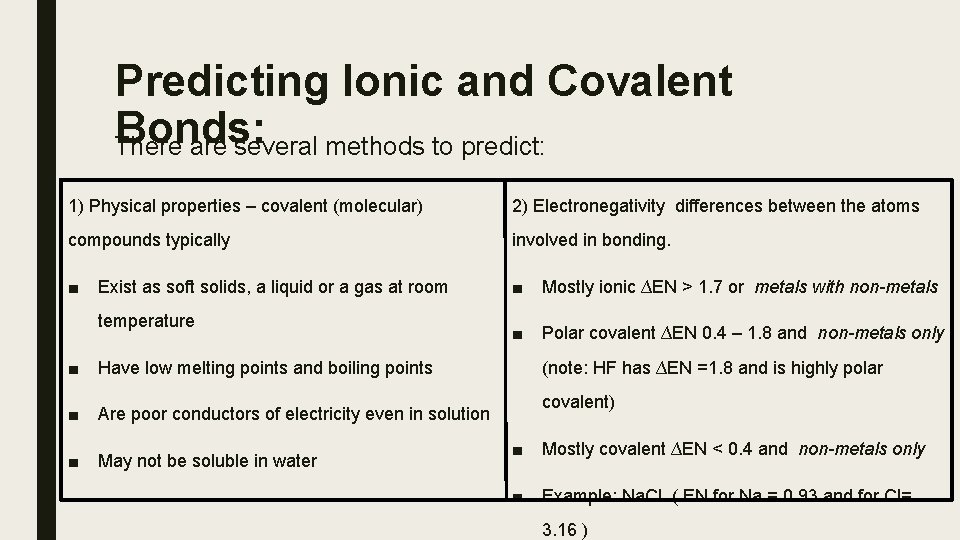
Predicting Ionic and Covalent Bonds: There are several methods to predict: 1) Physical properties – covalent (molecular) 2) Electronegativity differences between the atoms compounds typically involved in bonding. ■ Exist as soft solids, a liquid or a gas at room ■ Mostly ionic ∆EN > 1. 7 or metals with non-metals temperature ■ Polar covalent ∆EN 0. 4 – 1. 8 and non-metals only ■ Have low melting points and boiling points ■ Are poor conductors of electricity even in solution ■ May not be soluble in water (note: HF has ∆EN =1. 8 and is highly polar covalent) ■ Mostly covalent ∆EN < 0. 4 and non-metals only ■ Example: Na. Cl ( EN for Na = 0. 93 and for Cl= 3. 16 )

Polar Bonds ∆EN is the principle factor that determines the type of chemical bonding between two atoms. When a bond between two atoms is polar covalent, the electrons are shared unequally. Because one atom is more electronegative than the second atom in the bond, it has a greater attraction for the electrons shared in the bond. It develops a partial negative charge ( d- ) and the atom that is less electronegative develops a partial positive charge ( d+ ). This polarity in a bond is called a dipole, and ∆EN is often referred to as the dipole moment. The greater the ∆EN, the greater the dipole moment.
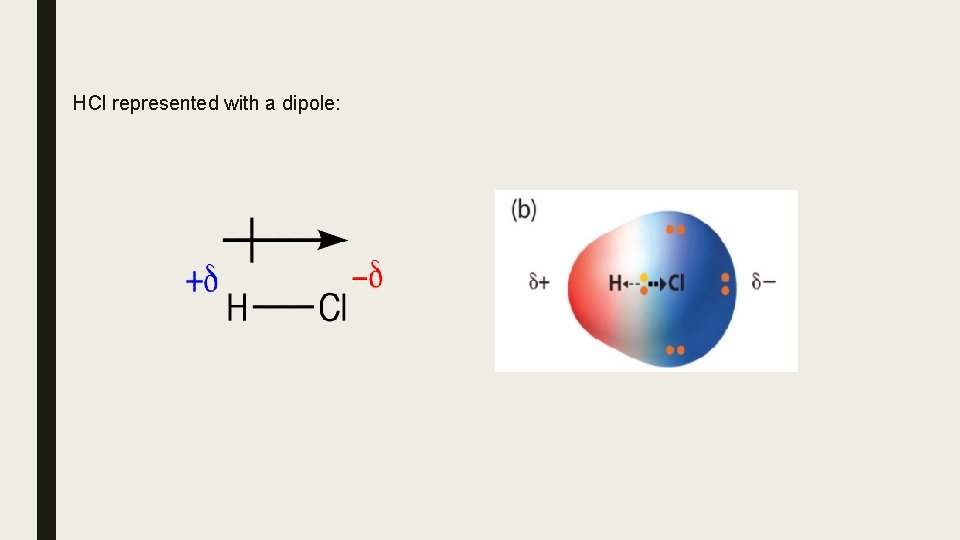
HCl represented with a dipole:
AP practice question

Covalent Bonding involves a balance between the forces of attraction and repulsion that act between the nuclei and electrons of two or more atoms.
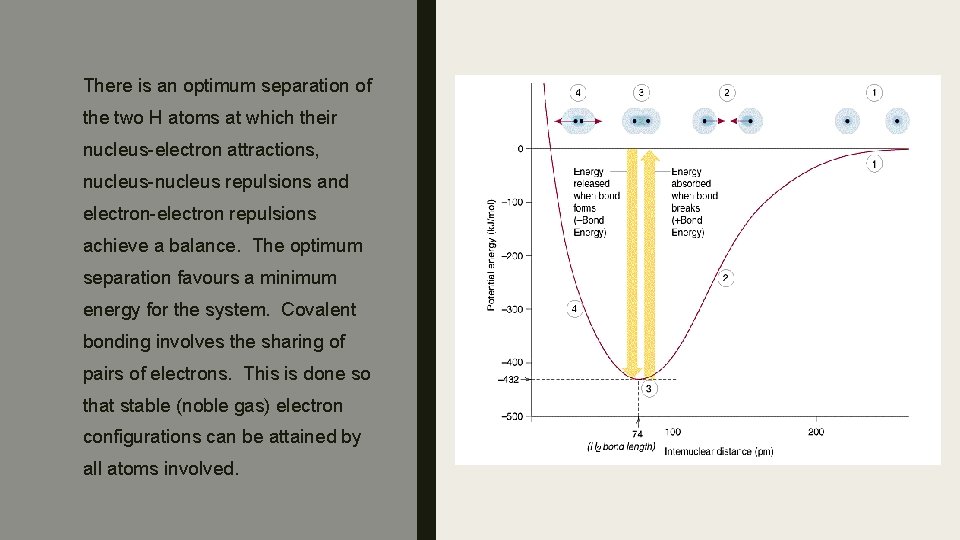
There is an optimum separation of the two H atoms at which their nucleus-electron attractions, nucleus-nucleus repulsions and electron-electron repulsions achieve a balance. The optimum separation favours a minimum energy for the system. Covalent bonding involves the sharing of pairs of electrons. This is done so that stable (noble gas) electron configurations can be attained by all atoms involved.

Quantum mechanical model can be applied here to explain the bonding in a covalent bond. A covalent bond may form when two half-filled atomic orbitals from two atoms overlap to share the same region of space. A covalent bond involves the formation of a new orbital, caused by the overlapping of atomic orbitals. The new orbital has energy levels that are lower than those of the original atomic orbitals. The new orbitals provide a more energetically favourable configuration than the two atoms had before they interacted.

Factors that influence optimal bond length: Bond order: Ø This is a measurement of the number of electrons held in between two atoms in a molecule. Most of the time it is equal to the number of bonds between the two atoms: a single bond has a bond order of 1, a triple bond has a bond order of 3, etc. Ø Generally, the higher bond order, the shorter the bond length Size of bonding atoms: Ø The larger the atom’s core (non-bonding) electron clouds, the further apart the nuclei of the bonding atoms, so the bond length is greater. Think of it this way: if an atom is large, it is because the valence electrons are not very attracted to the nucleus likely due to shielding from the core electrons. Therefore, the bonded valence electrons will also not experience a very strong attraction to the nucleus.

AP practice questions
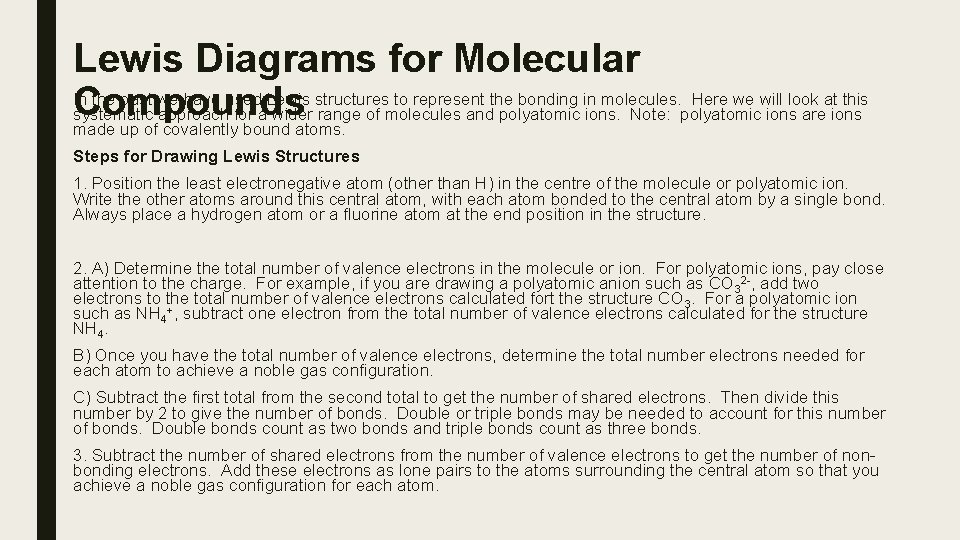
Lewis Diagrams for Molecular In the past we have used Lewis structures to represent the bonding in molecules. Here we will look at this Compounds systematic approach for a wider range of molecules and polyatomic ions. Note: polyatomic ions are ions made up of covalently bound atoms. Steps for Drawing Lewis Structures 1. Position the least electronegative atom (other than H) in the centre of the molecule or polyatomic ion. Write the other atoms around this central atom, with each atom bonded to the central atom by a single bond. Always place a hydrogen atom or a fluorine atom at the end position in the structure. 2. A) Determine the total number of valence electrons in the molecule or ion. For polyatomic ions, pay close attention to the charge. For example, if you are drawing a polyatomic anion such as CO 32 -, add two electrons to the total number of valence electrons calculated fort the structure CO 3. For a polyatomic ion such as NH 4+, subtract one electron from the total number of valence electrons calculated for the structure NH 4. B) Once you have the total number of valence electrons, determine the total number electrons needed for each atom to achieve a noble gas configuration. C) Subtract the first total from the second total to get the number of shared electrons. Then divide this number by 2 to give the number of bonds. Double or triple bonds may be needed to account for this number of bonds. Double bonds count as two bonds and triple bonds count as three bonds. 3. Subtract the number of shared electrons from the number of valence electrons to get the number of nonbonding electrons. Add these electrons as lone pairs to the atoms surrounding the central atom so that you achieve a noble gas configuration for each atom.

■ SO 2


Coordinate Covalent Bonds Co-ordinate covalent bond: a bond in which both bonding electrons are provided by one of the atoms Lewis Structures that include a co-ordinate covalent bond: Example: NH 4+ Another example: H 3 O+

Central Atoms with an Expanded Valence Level The octet rule allows for a maximum of 4 bonds around an atom. Some atoms can hold more than the eight electrons in the valence of the central atom. The central atom is said to have an expanded valence energy level. Example: PCl 5 An another: Xe. F 2

Method for drawing structures with expanded valence shell: 1. Put least electronegative atom in the centre. 2. Surround it with the other atoms and draw in a bond to each one. 3. Calculate how many valence electrons are involved and surround each of the more electronegative atoms with enough electrons to give them octets. 4. Put any remaining electrons onto the central atom.

■ SBr 6

■ ICl 3


Other compounds and ions that do not obey the octet rule: Boron and Beryllium compounds: a) BCl 3 b) Be. Cl 2

Other compounds and ions that do not obey the octet rule: Phosphate and sulfate ions: a) SO 42 - b) PO 43 -

Other compounds and ions that do not obey the octet rule: Free radicals For example: NO 2

Resonance Structures Sometimes more than one valid Lewis structure is possible for a given molecule. Example: O 3 Given this structure, you would expect 1 longer bond lengths for -O and one shorter length for the tighter O=O bond. Experimentally what is found is only one bond length and strength which is between those expected for the single and double bonds. We assume that the correct depiction is given only by the superposition of both. Resonance occurs when more than one valid Lewis structure can be written for a particular molecule. This is represented by .

■ CO 32 -
AP practice questions
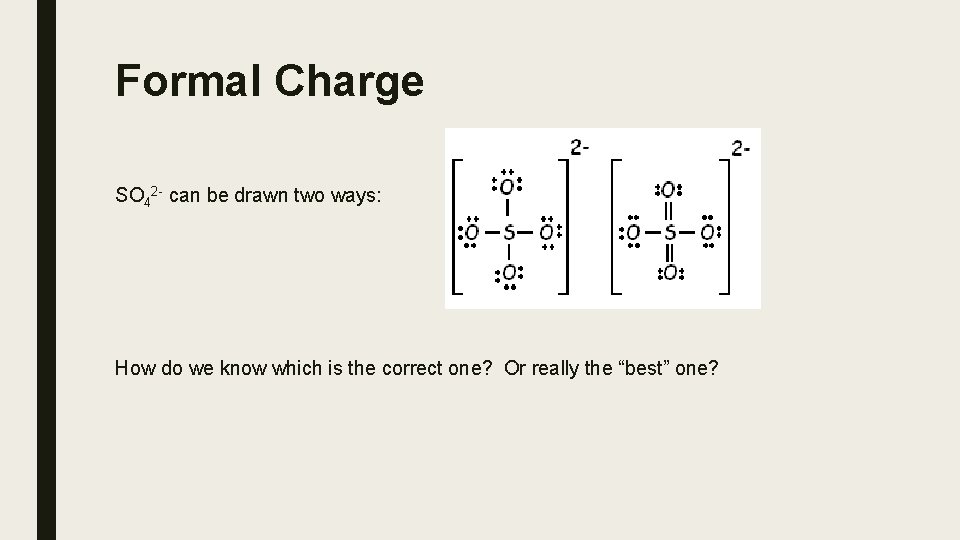
Formal Charge SO 42 - can be drawn two ways: How do we know which is the correct one? Or really the “best” one?

Often, many Lewis dot structures are possible. These are possible resonance structures, but often we should write the one which is most stable. The formal charge acts as a guide to the stability of the dot structure. The formal charge is a hypothetical charge from the dot structure. Formal charge rules are: ■ Formal charge of an atom = (valence e-) – (½ bonding e-) - (lone e-) ■ Atoms in a molecule try to have formal charges as close to zero as possible. ■ More electronegative atoms should have negative formal charges. ■ Adjacent atoms should have opposite formal charges. ■ The sum of the formal charges of all atoms in the structure must equal the overall charge on that species.
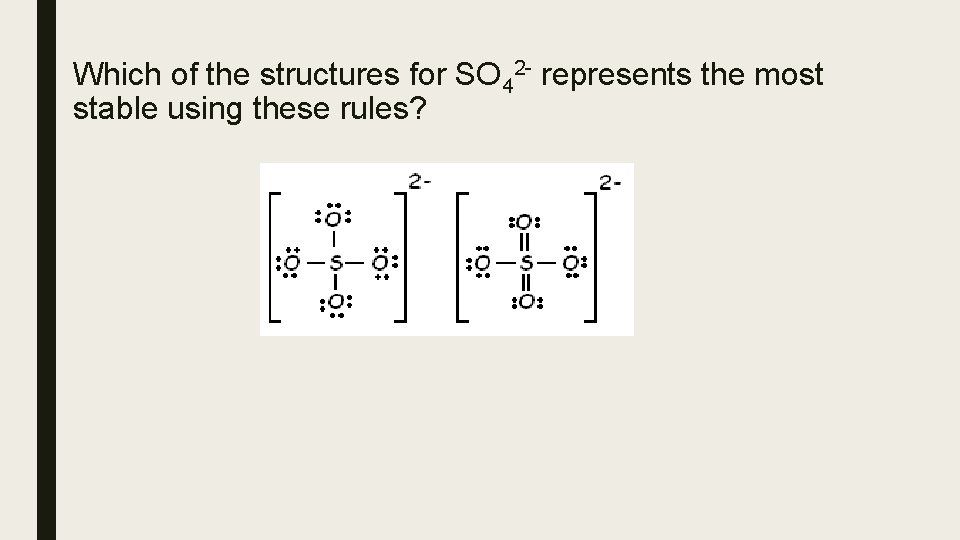
Which of the structures for SO 42 - represents the most stable using these rules?

Note: SO 2

AP practice question
- Slides: 31