Bonding Molecular Structure C 3 6 Use structural
Bonding & Molecular Structure C. 3. 6 Use structural formulas of hydrocarbons to illustrate carbon's ability to form single and multiple bonds within a molecule.

What are hydrocarbons? • Compounds composed of hydrogens and carbons only. • Organic Chemistry studies Carbon Chemistry exclusively and therefore hydrocarbons are very popular reactions to study.

Hydrocarbons & the Petroleum Industry • Most of the wars between the US & the Middle East is over control of hydrocarbons. • These hydrocarbons are petroleum, gas, and oil deposits from super dead & super old (thousands of years) sea creatures decomposed & buried by geological activity. • Hydrocarbons can form single, double, and triple bonds. • Because of their unique chemical & physical properties hydrocarbons are valuable to extract energy from in the form of combustion reactions. • Carbon & Hydrogen are both critical elements to all life as we understand it.
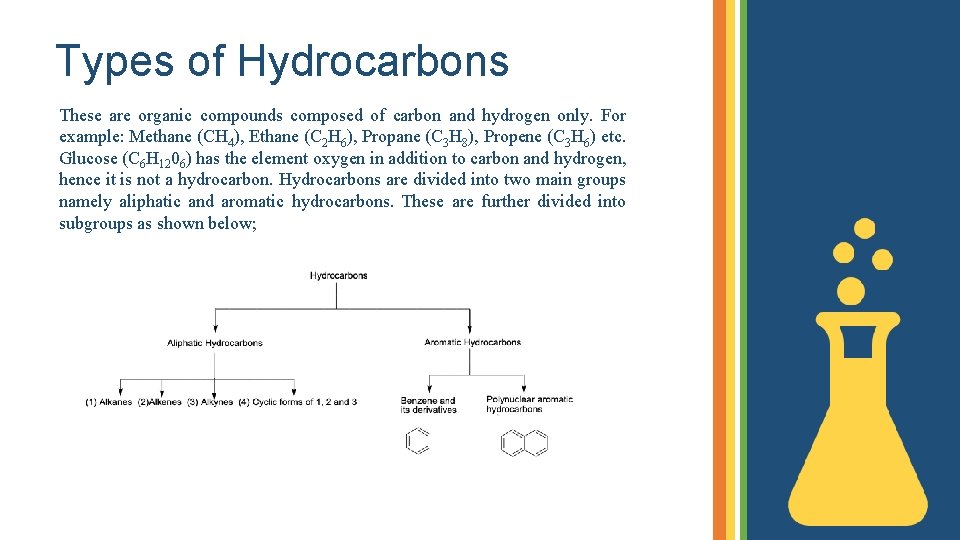
Types of Hydrocarbons These are organic compounds composed of carbon and hydrogen only. For example: Methane (CH 4), Ethane (C 2 H 6), Propane (C 3 H 8), Propene (C 3 H 6) etc. Glucose (C 6 H 1206) has the element oxygen in addition to carbon and hydrogen, hence it is not a hydrocarbon. Hydrocarbons are divided into two main groups namely aliphatic and aromatic hydrocarbons. These are further divided into subgroups as shown below;
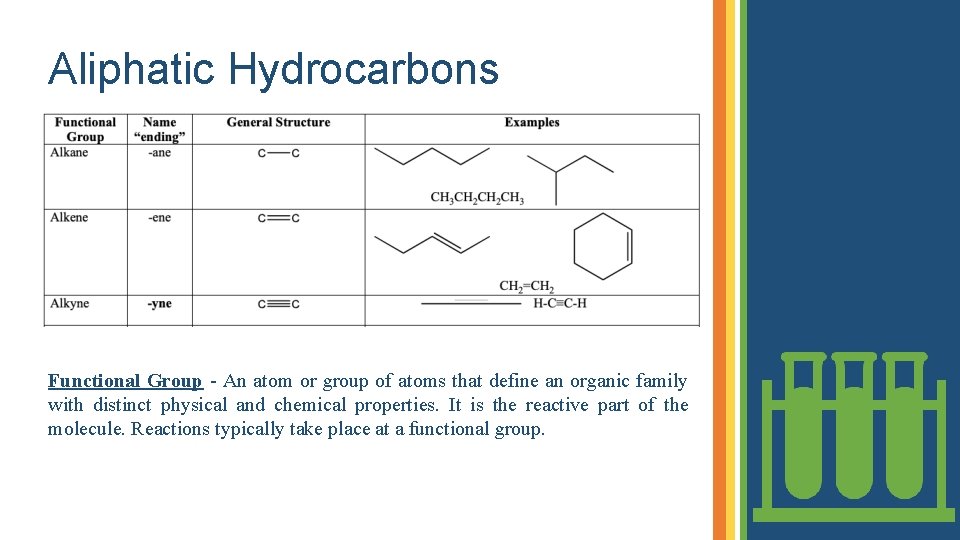
Aliphatic Hydrocarbons Functional Group - An atom or group of atoms that define an organic family with distinct physical and chemical properties. It is the reactive part of the molecule. Reactions typically take place at a functional group.
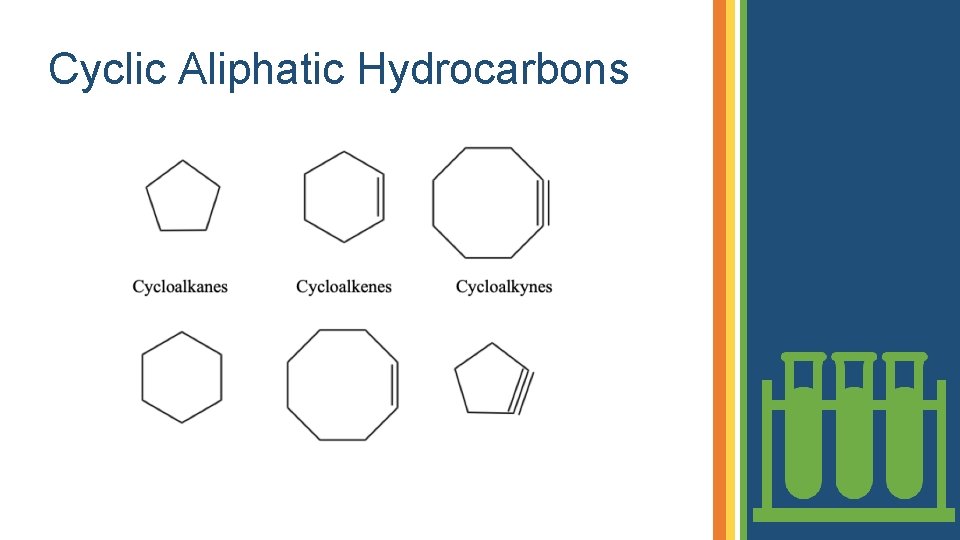
Cyclic Aliphatic Hydrocarbons

Aromatic Hydrocarbons (AKA - Arenes; AKA - Aryl Hydrocarbons) • Any hydrocarbon that keeps alternating double bonds throughout its entire structure. • These alternating double bonds act like train tracks that allow the electrons to delocalize or move around freely within the structure.
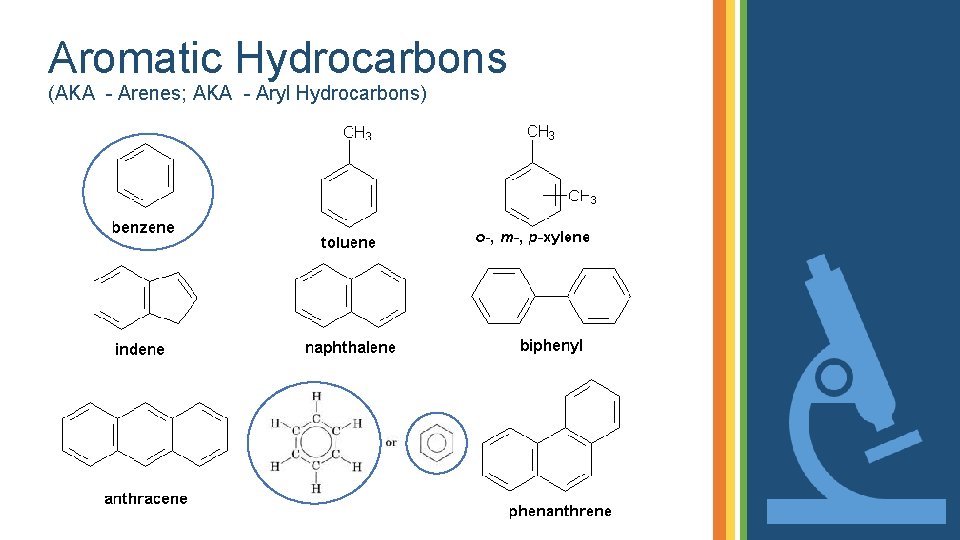
Aromatic Hydrocarbons (AKA - Arenes; AKA - Aryl Hydrocarbons)

Time to Draw & Synthesize! Chem-is-try
- Slides: 9