Bonding Ionic Covalent Metallic How do atoms bondjoin
Bonding Ionic Covalent (Metallic)

How do atoms bond(join) together to form the millions of different compounds that make up the world? It all comes down to the electrons!
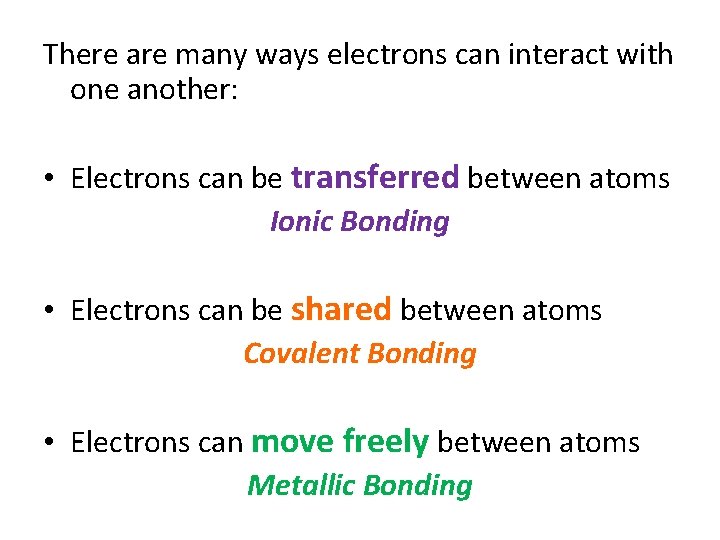
There are many ways electrons can interact with one another: • Electrons can be transferred between atoms Ionic Bonding • Electrons can be shared between atoms Covalent Bonding • Electrons can move freely between atoms Metallic Bonding
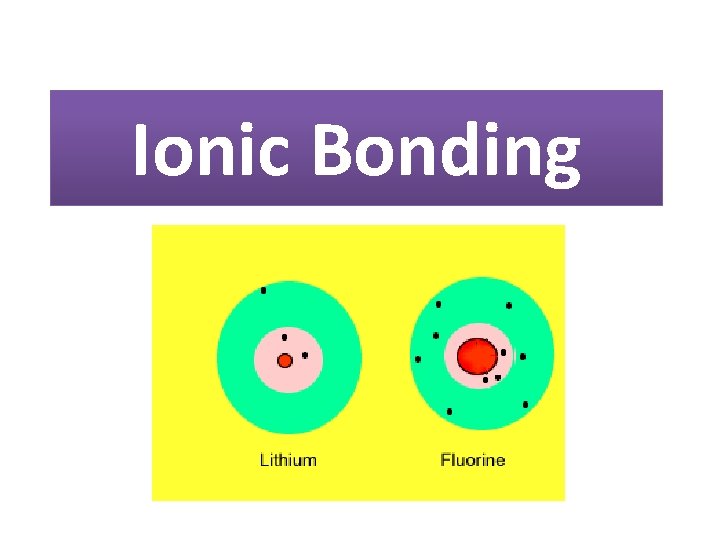
Ionic Bonding
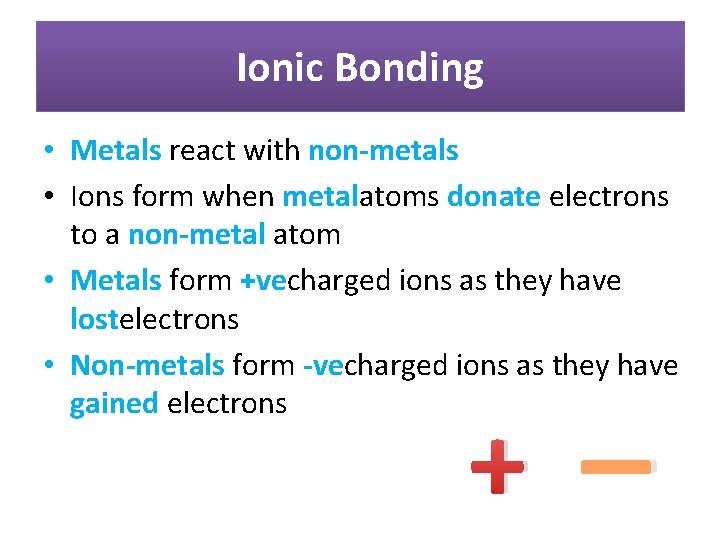
Ionic Bonding • Metals react with non-metals • Ions form when metalatoms donate electrons to a non-metal atom • Metals form +vecharged ions as they have lostelectrons • Non-metals form -vecharged ions as they have gained electrons _ +
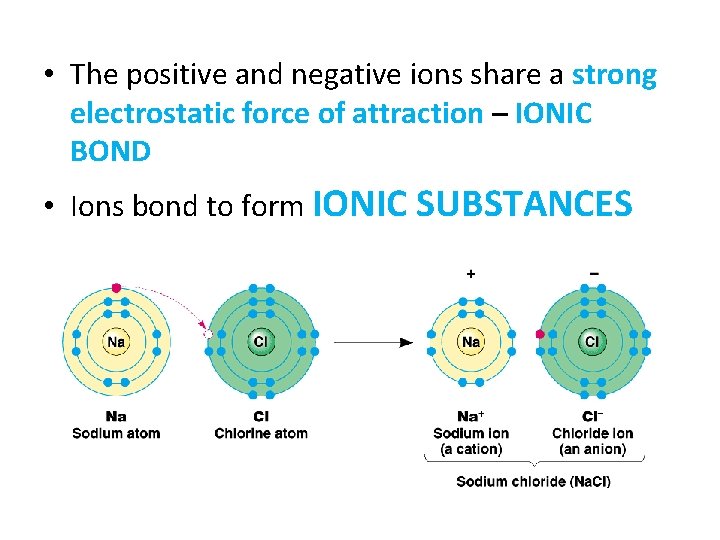
• The positive and negative ions share a strong electrostatic force of attraction – IONIC BOND • Ions bond to form IONIC SUBSTANCES
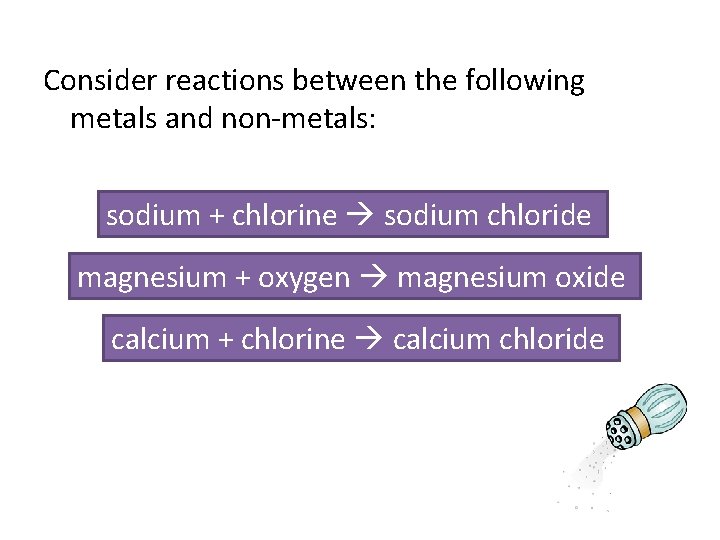
Consider reactions between the following metals and non-metals: sodium + chlorine sodium chloride magnesium + oxygen magnesium oxide calcium + chlorine calcium chloride
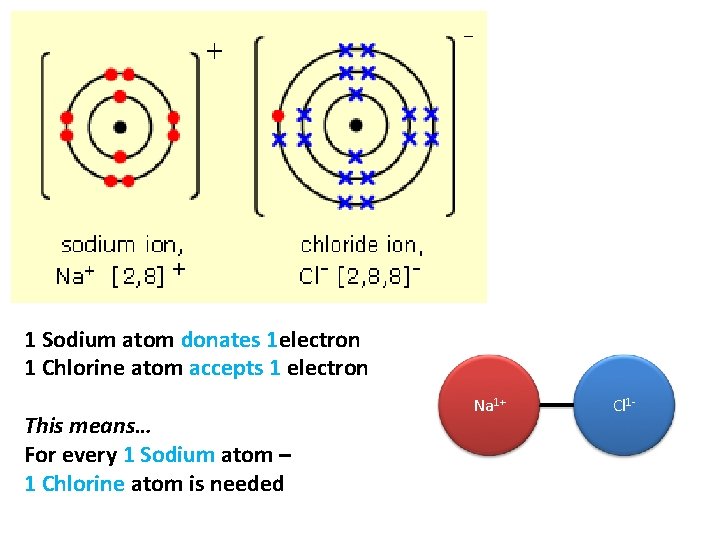
1 Sodium atom donates 1 electron 1 Chlorine atom accepts 1 electron This means… For every 1 Sodium atom – 1 Chlorine atom is needed Na 1+ Cl 1 -
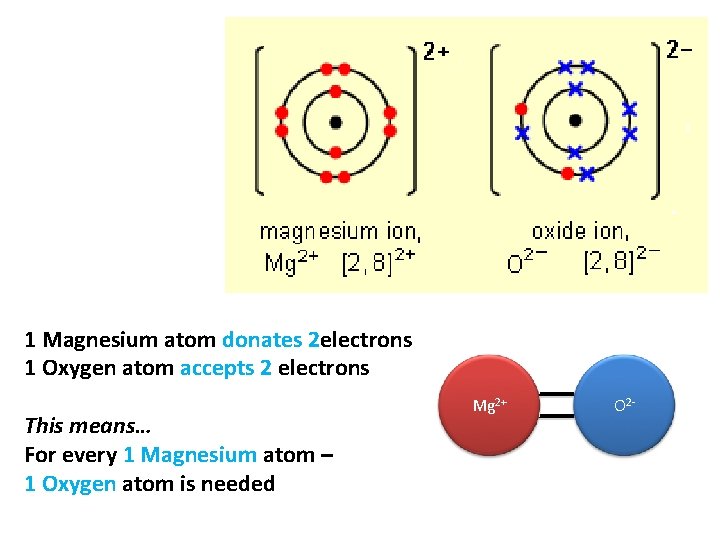
1 Magnesium atom donates 2 electrons 1 Oxygen atom accepts 2 electrons This means… For every 1 Magnesium atom – 1 Oxygen atom is needed Mg 2+ O 2 -
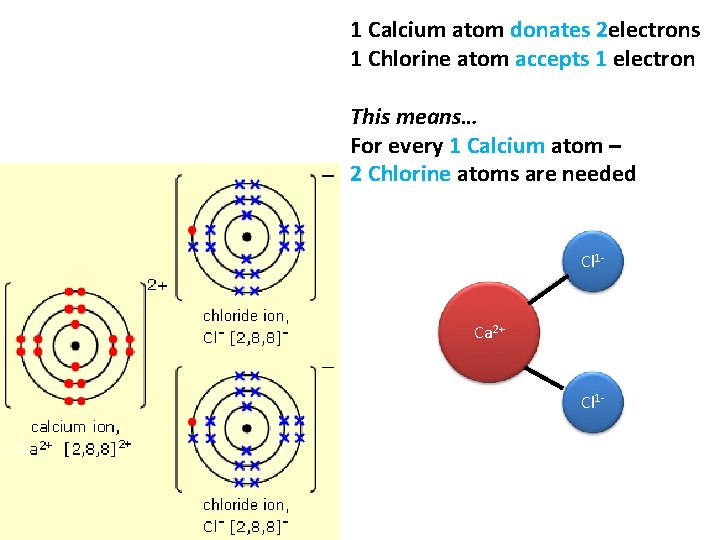
1 Calcium atom donates 2 electrons 1 Chlorine atom accepts 1 electron This means… For every 1 Calcium atom – 2 Chlorine atoms are needed Cl 1 - Ca 2+ Cl 1 -

Have you noticed how electrons are arranged within the shells? Individually? ? In pairs? ? In groups? ?
Ionic Lattice • Not just a pairof ions • Many atoms (ions) bond to form an IONIC LATTICE • The number of positive and negative ions vary, depending on how many electrons are transferred between the metal and non-metal.
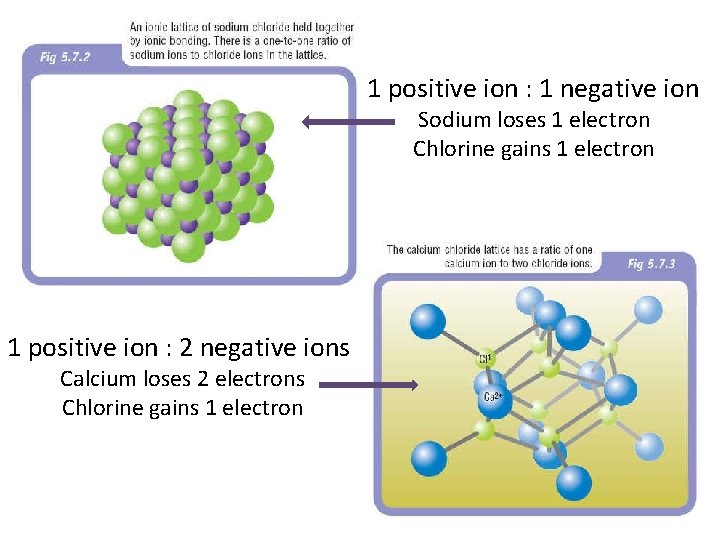
1 positive ion : 1 negative ion Sodium loses 1 electron Chlorine gains 1 electron 1 positive ion : 2 negative ions Calcium loses 2 electrons Chlorine gains 1 electron

Properties of Ionic Substances The very strong ionic bonds within the lattice means… • HIGH melting point (usually over 250 o. C) • HARD– when apply force the force is spread throughout lattice • BRITTLE– large force can cause ions to move, therefore repel one another lattice breaks
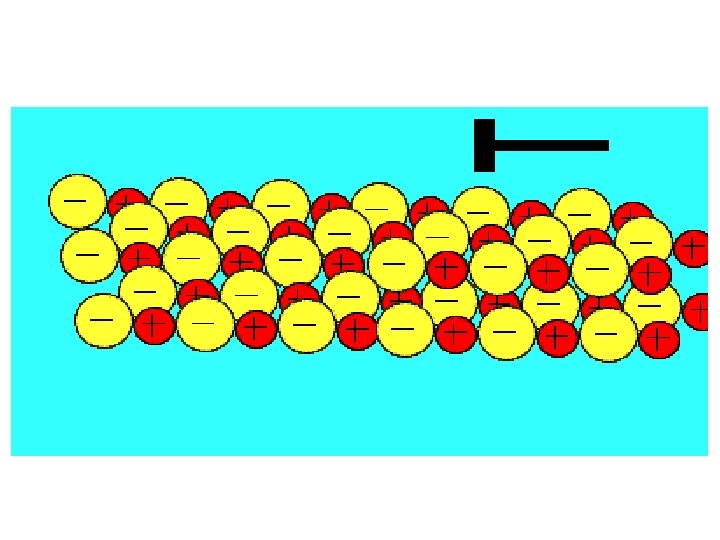
Properties of Ionic Substances The very strong ionic bonds within the lattice means… • DO NOT conduct ELECTRICITY when solid – ions are not free to move • WILL conduct ELECTRICITY when in aqueous solution– ions are now free to move

Examples of Ionic Substances Sodium Chloride Magnesium Oxide Calcium Chloride Can you think of some others? . . .
Covalent Bonding
Covalent Bonding • Most compounds (substances) in the world are formed through covalent bonding • Non-metals react with non-metals • Atoms shareapairofelectrons– this is called a COVALENT BOND • Two types of covalent bonds: Covalent Molecular ANDCovalent Network
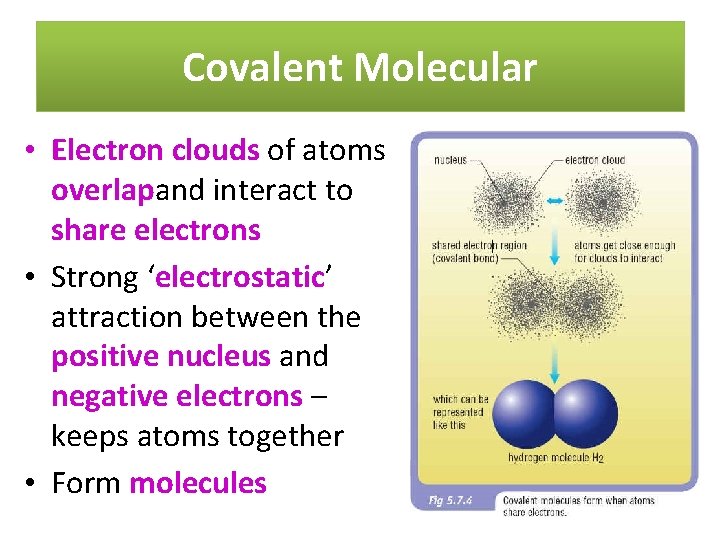
Covalent Molecular • Electron clouds of atoms overlapand interact to share electrons • Strong ‘electrostatic’ attraction between the positive nucleus and negative electrons – keeps atoms together • Form molecules
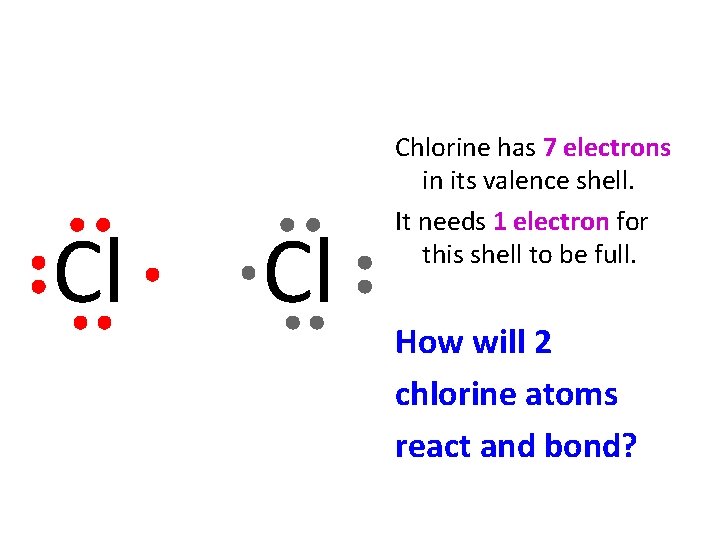
Cl Cl Chlorine has 7 electrons in its valence shell. It needs 1 electron for this shell to be full. How will 2 chlorine atoms react and bond?

Cl Cl Each atom of Chlorine achieves a full valence shell by sharing the 2 electrons in the middle.
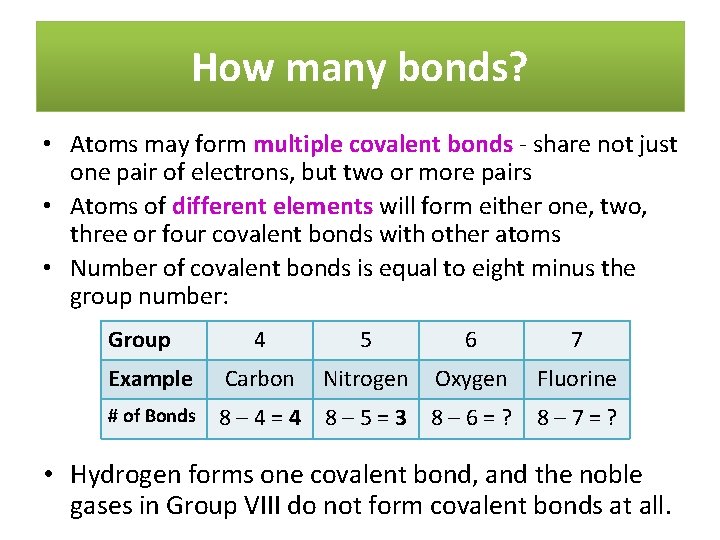
How many bonds? • Atoms may form multiple covalent bonds - share not just one pair of electrons, but two or more pairs • Atoms of different elements will form either one, two, three or four covalent bonds with other atoms • Number of covalent bonds is equal to eight minus the group number: Group 4 5 6 7 Example Carbon Nitrogen Oxygen Fluorine # of Bonds 8– 4=4 8– 5=3 8– 6=? 8– 7=? • Hydrogen forms one covalent bond, and the noble gases in Group VIII do not form covalent bonds at all.
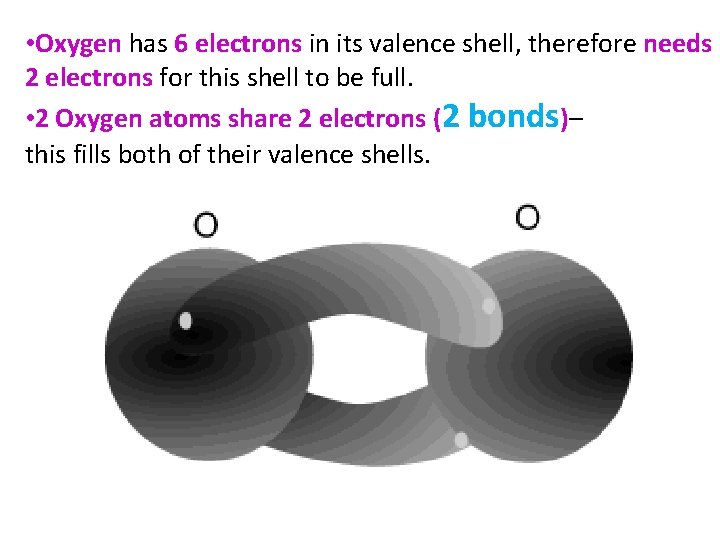
• Oxygen has 6 electrons in its valence shell, therefore needs 2 electrons for this shell to be full. • 2 Oxygen atoms share 2 electrons (2 bonds)– this fills both of their valence shells.
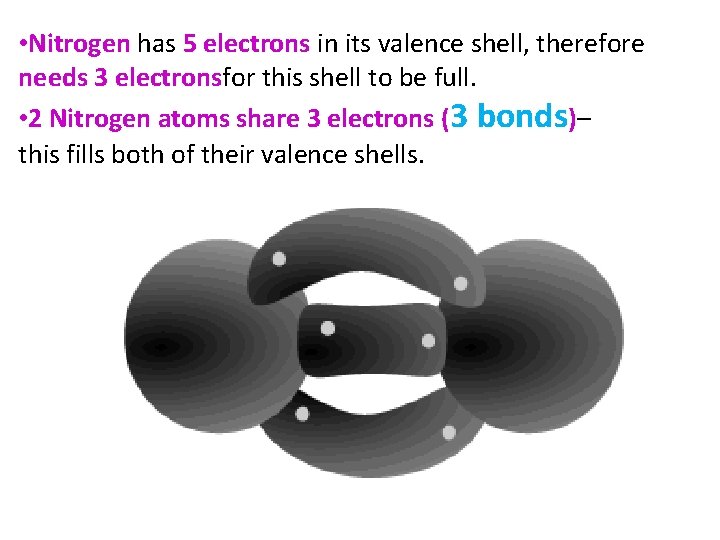
• Nitrogen has 5 electrons in its valence shell, therefore needs 3 electronsfor this shell to be full. • 2 Nitrogen atoms share 3 electrons (3 bonds)– this fills both of their valence shells.
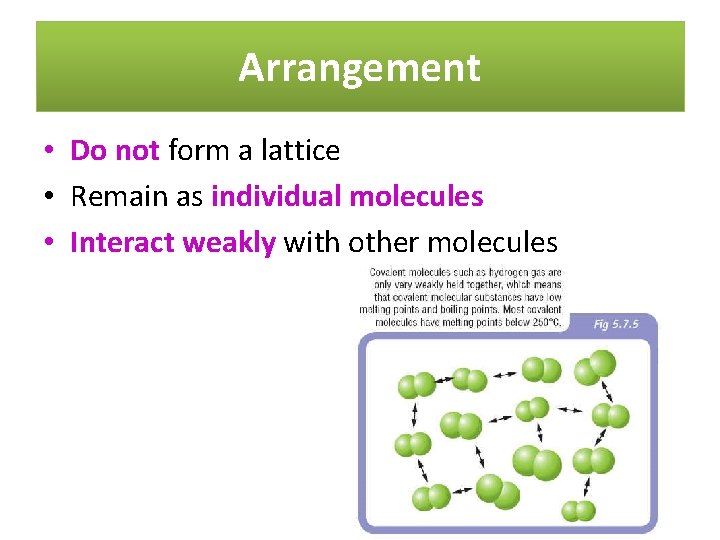
Arrangement • Do not form a lattice • Remain as individual molecules • Interact weakly with other molecules
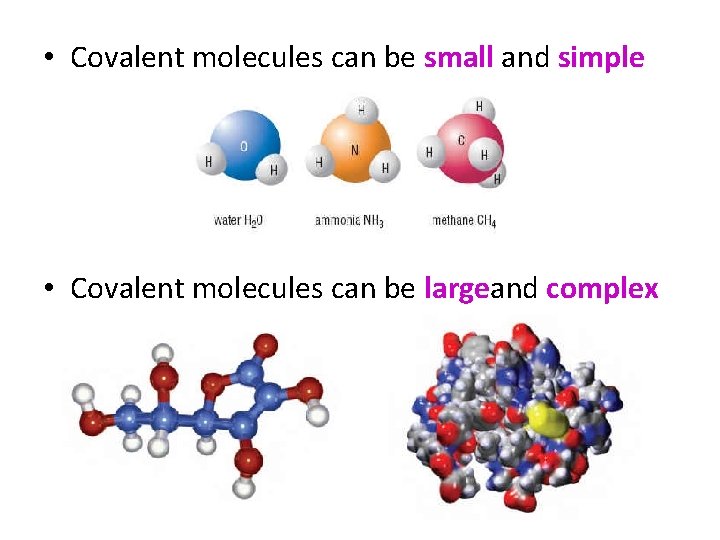
• Covalent molecules can be small and simple • Covalent molecules can be largeand complex
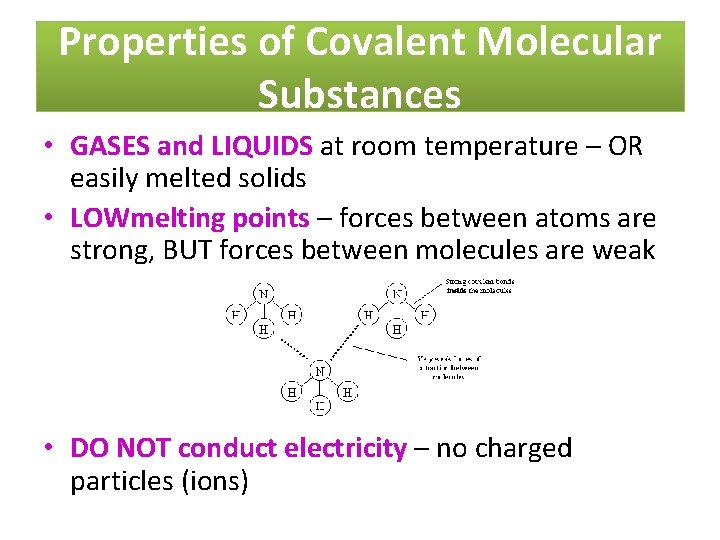
Properties of Covalent Molecular Substances • GASES and LIQUIDS at room temperature – OR easily melted solids • LOWmelting points – forces between atoms are strong, BUT forces between molecules are weak • DO NOT conduct electricity – no charged particles (ions)
Covalent Network • Do not exist as individual molecules • Form giant networks of covalently bonded atoms • Carbon (C) and Silicon (Si)
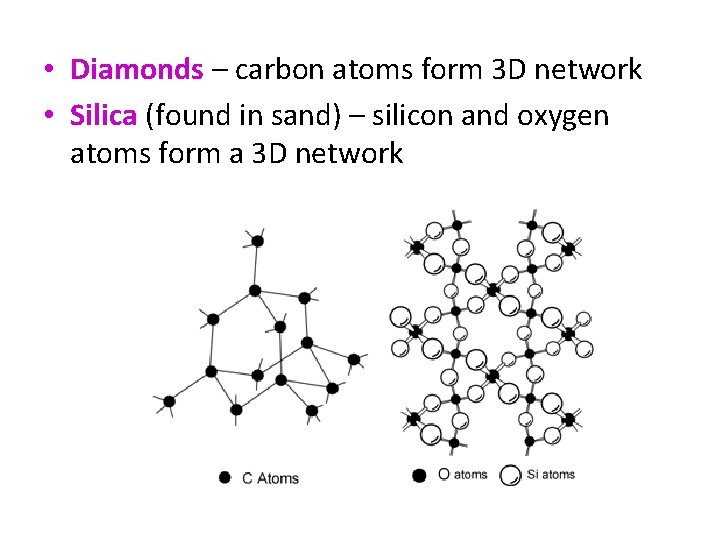
• Diamonds – carbon atoms form 3 D network • Silica (found in sand) – silicon and oxygen atoms form a 3 D network
Properties of Covalent Network Substances • HARD and BRITTLE • HIGH melting points – strong covalent bonds between molecules in network ** Diamonds melt at just over 4000 o. C! • DO NOTconduct ELECTRICITY – no charged particles (ions) • INSOLUBLE in water – the bonds will not break if you add substance to water • Non-reactors

Examples of Covalent Substances Carbon Dioxide Methane Diamonds Can you think of some others? . . .
- Slides: 33